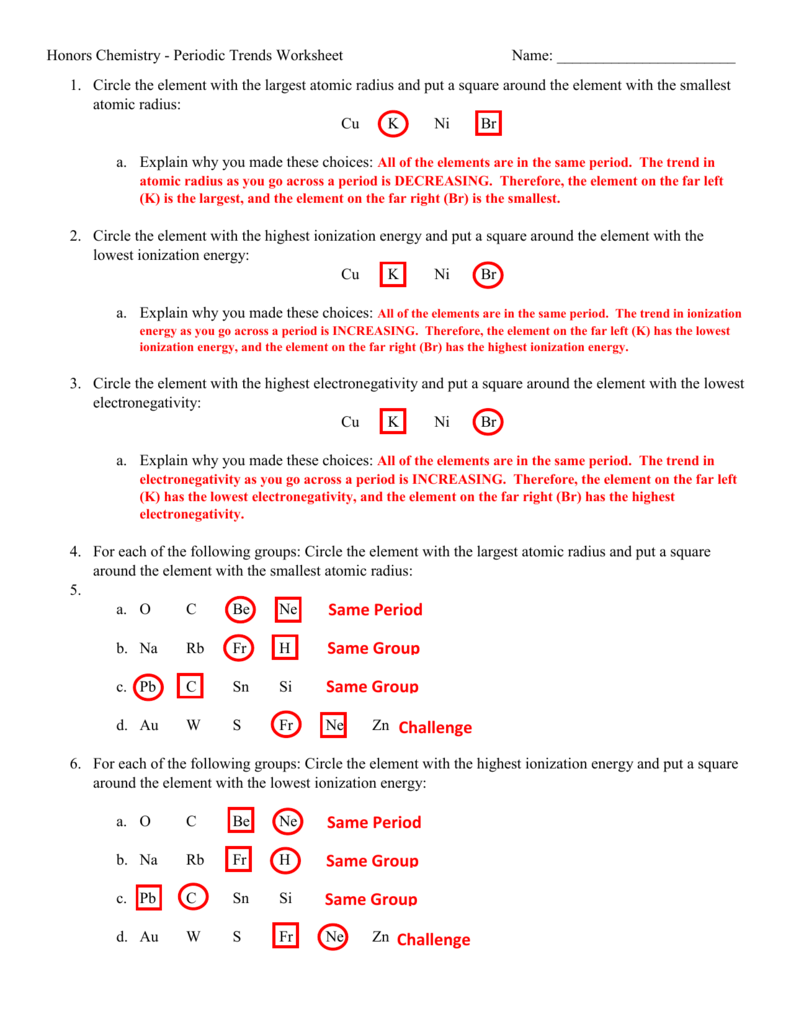
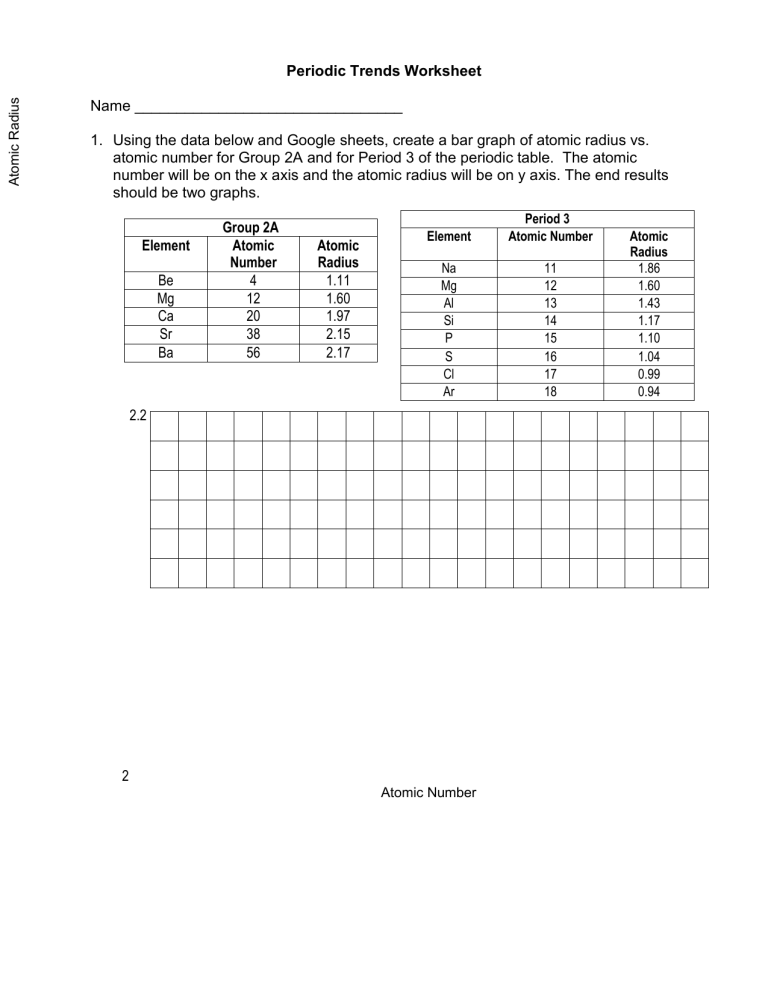
Chemistry Periodic Trends Worksheet Answers - Understand why some acids dissolve in water to make acidic solution, while others dissolve in water to make basic solutions. Web chemistry periodic trends worksheet copy flashcards | quizlet. Web what is the periodic trend for atomic size from top to bottom in a group? Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and. A) li or k b) ca or ni c) ga or b d) o or c e) cl or br f) be or ba g) si or s. From left to right in a period? Atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. Which atom in each pair has the larger atomic radius? Web the periodic trends chemistry worksheet will help you to learn and understand what a periodic trend is. Know periodic trends of atomic size, ionic size, ionization energy, and electron affinity.
Periodic Trends Practice Worksheet
Know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. From left to right in a period? Use your notes to answer the following questions. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,. Web a summary worksheet activity of the periodic table trends including:
Periodic Trends Worksheet Answers
Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,. Web periodic trends answer key vocabulary: Normalized probability function of the 1s, 2s, and 2p orbitals illustrates orbital. Web explain why you made these choices:all of the elements are in the same period. Understand the reasons for metallic, nonmetallic, and metalloid character.
Periodic Trends Worksheet Answers
Web major periodic trends include: Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,. Which atom in each pair has the. Web what is the periodic trend for atomic size from top to bottom in a group? A) li or k b) ca or ni c) ga or b d) o.
Electron Configuration Chem Worksheet 5 6 Answers
Web explain why you made these choices:all of the elements are in the same period. Visual summary of periodic trends figure 1. In this periodic trends worksheet with answers, we will look at. Electronegativity, electron affinity, ionization energy, ionic. The # of protons increase, so electrons are more attracted.
Periodic Table Trends Worksheet Answers Chemistry A Study Of Matter
Atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and. Web explain why you made these choices:all of the elements are in the same period. The trend in ionization energy as you go. Visual summary of periodic trends figure 1.
Chemistry Honors Periodic Trends Worksheet
Web periodic trends answer key vocabulary: Use the periodic table and your knowledge of periodic trends to answer the following questions. Which atom in each pair has the. Web many of the trends in the periodic table are useful tools for predicting electronic properties and chemical. In this periodic trends worksheet with answers, we will look at.
Worksheet On Periodic Trends
Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,. Web the periodic trends chemistry worksheet will help you to learn and understand what a periodic trend is. Electronegativity, electron affinity, ionization energy, ionic. Understand why some acids dissolve in water to make acidic solution, while others dissolve in water to make.
PERIODIC TRENDS WORKSHEET ANSWERS
Atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. Which atom in each pair has the. From left to right in a period? Web periodic trends worksheet 4.8 (4 reviews) rank the following elements by increasing atomic radius: Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,.
Worksheet On Periodic Trends
Estimate the ionization energy value for each missing element in. Electronegativity, electron affinity, ionization energy, ionic. Web periodic trends worksheet 4.8 (4 reviews) rank the following elements by increasing atomic radius: Web the periodic trends chemistry worksheet will help you to learn and understand what a periodic trend is. Know periodic trends of atomic size, ionic size, ionization energy, and.
Periodic Table Worksheet Answer Key Pdf Periodic Table Worksheet
Web the periodic trends chemistry worksheet will help you to learn and understand what a periodic trend is. Visual summary of periodic trends figure 1. Web atomic radius trends on periodic table. Web periodic trends worksheet 4.8 (4 reviews) rank the following elements by increasing atomic radius: Web explain why you made these choices:all of the elements are in the.
In this periodic trends worksheet with answers, we will look at. Estimate the ionization energy value for each missing element in. Use your notes to answer the following questions. From left to right in a period? Web chemistry periodic trends worksheet copy flashcards | quizlet. Web periodic trends answer key vocabulary: Web many of the trends in the periodic table are useful tools for predicting electronic properties and chemical. Web what is the periodic trend for atomic size from top to bottom in a group? Web major periodic trends include: Electronegativity, electron affinity, ionization energy, ionic. Use the periodic table and your knowledge of periodic trends to answer the following questions. Atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. Understand the reasons for metallic, nonmetallic, and metalloid character. Normalized probability function of the 1s, 2s, and 2p orbitals illustrates orbital. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and. Ionization energy by shari kendrick. Which atom in each pair has the larger atomic radius? Know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. Visual summary of periodic trends figure 1. Web atomic radius trends on periodic table.
Use Your Notes To Answer The Following Questions.
Web major periodic trends include: Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering the periodic trends,. Web periodic trends answer key vocabulary: Use the periodic table and your knowledge of periodic trends to answer the following questions.
Understand The Reasons For Metallic, Nonmetallic, And Metalloid Character.
The # of protons increase, so electrons are more attracted. Web the periodic trends chemistry worksheet will help you to learn and understand what a periodic trend is. In this periodic trends worksheet with answers, we will look at. Understand why some acids dissolve in water to make acidic solution, while others dissolve in water to make basic solutions.
Study With Quizlet And Memorize Flashcards Containing Terms.
Web many of the trends in the periodic table are useful tools for predicting electronic properties and chemical. Ionization energy by shari kendrick. A) li or k b) ca or ni c) ga or b d) o or c e) cl or br f) be or ba g) si or s. Visual summary of periodic trends figure 1.
Web Atomic Radius Trends On Periodic Table.
Estimate the ionization energy value for each missing element in. It will also explain the various trends of. Web what is the periodic trend for atomic size from top to bottom in a group? Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and.