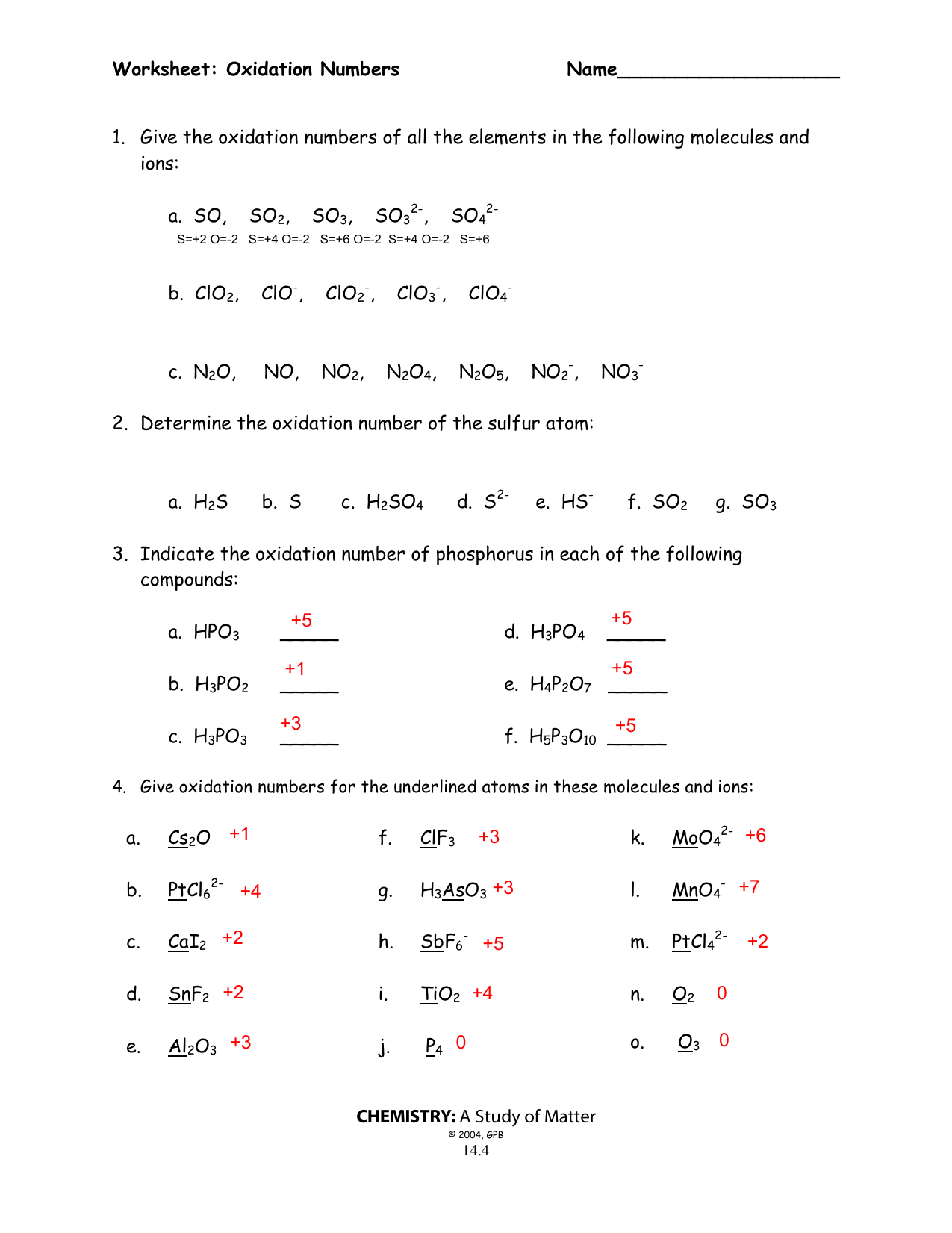
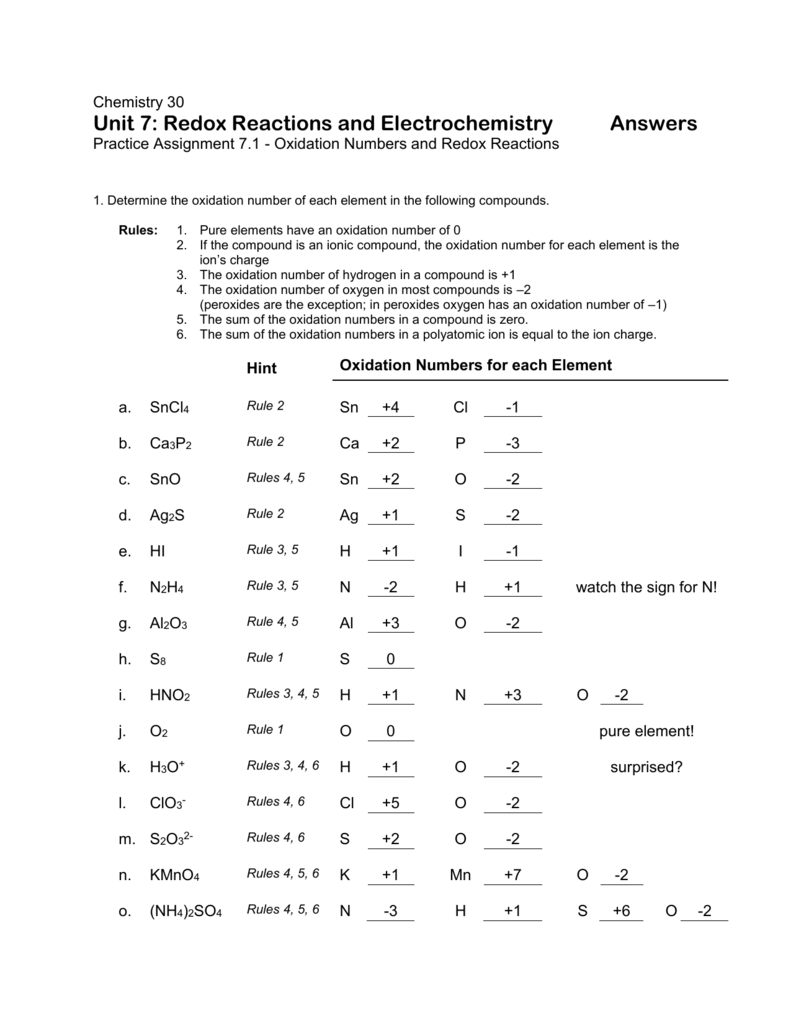
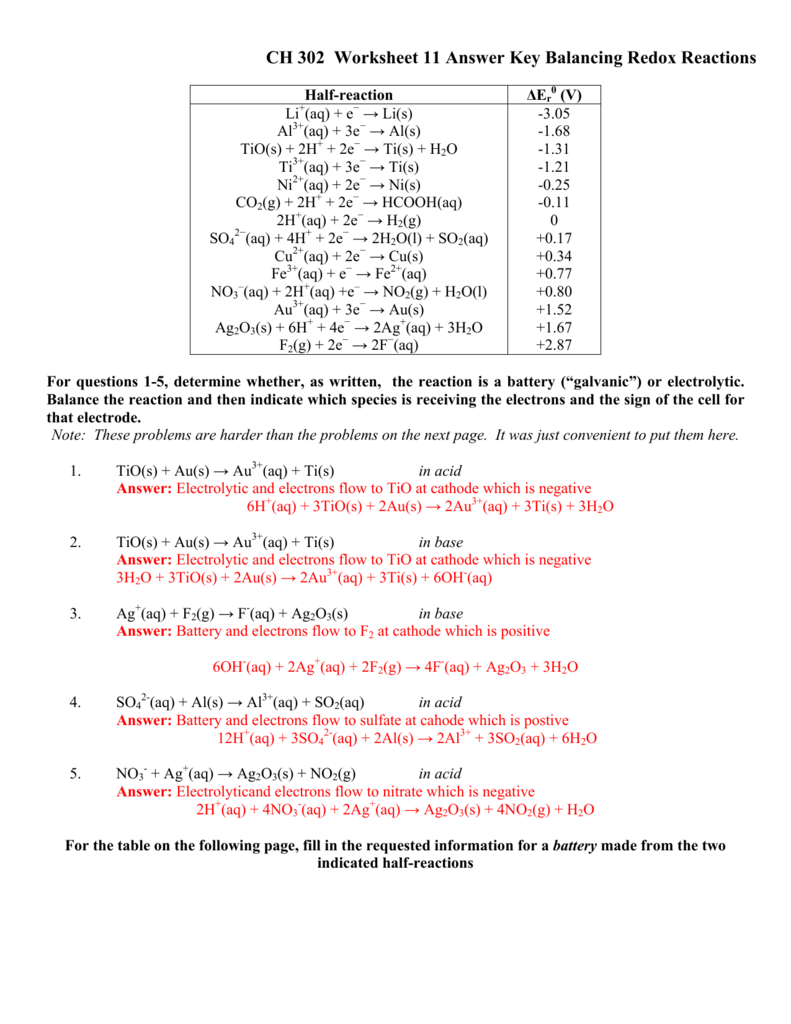
Worksheet Oxidation Numbers Answer Key - The handout outlines and illustrates the rules for assigning oxidation numbers. Na2o + h2o g 2 naoh. Web rules for assigning oxidation numbers 1. Web this product contains two pages. Oxidation numbers hey name 1. Give the oxidation numbers of all the elements in the following molecules and ions: The charge on all free elements is zero. Web assign oxidation numbers to each of the atoms in the following compounds: The oxidation number chemistry worksheet contains. The oxidation number of an element in a monatomic ion equals the charge of the.
Worksheet Oxidation Numbers Answer Key
Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a. The oxidation number of an element in a monatomic ion equals the charge of the. Identify each of the following as examples of oxidation of reduction: Web this product contains two pages. Basic oxide of nonacidic cation gives hydroxide in.
Worksheet Oxidation Numbers Answer Key Ivuyteq
The oxidation number of an element in a. Give the oxidation numbers of all the elements in the following. I2o5 + h2o g 2 hio 3. Web use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. Web rules for assigning oxidation numbers 1.
Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume
Web identify the species being oxidised and reduced in each of the following reactions and write their half reactions: The oxidation number chemistry worksheet contains. Web give the oxidation numbers of all the elements in the following molecules and ions: A) li(s) li+(aq) + e−= oxidation b) s(s) + 2e−s2−(aq) = reduction c) cr3+(aq) +. Na2o + h2o g 2.
41 Oxidation Number Worksheet Answer Key Worksheet Master
Web rules for assigning oxidation numbers 1. Web give the oxidation numbers of all the elements in the following molecules and ions: The oxidation number of a monatomic ion equals the. Web assign oxidation numbers to each of the atoms in the following compounds: Amazing activity to practice the oxidation numbers.
️Ap Chemistry Redox Reactions Worksheet Free Download Goodimg.co
The oxidation number of any uncombined element is 0. Basic oxide of nonacidic cation gives hydroxide in water. The oxidation number of an element in a monatomic ion equals the charge of the. The charge on all metals of group 1 of the periodic table is +1 c. Web this product contains two pages.
Worksheet Assigning Oxidation Numbers Key
Web instructions on how to use the oxidation number chemistry worksheet. Na2o + h2o g 2 naoh. Web this product contains two pages. Web give the oxidation numbers of all the elements in the following molecules and ions: Identify each of the following as examples of oxidation of reduction:
Worksheet Oxidation Numbers Answer Key Ivuyteq
Amazing activity to practice the oxidation numbers. Web instructions on how to use the oxidation number chemistry worksheet. A pure element has an oxidation number of 0. Web identify the species being oxidised and reduced in each of the following reactions and write their half reactions: A) li(s) li+(aq) + e−= oxidation b) s(s) + 2e−s2−(aq) = reduction c) cr3+(aq).
Oxidation And Reduction Worksheet
Web assign oxidation numbers to each of the atoms in the following compounds: Identify each of the following as examples of oxidation of reduction: This brief quiz and worksheet will assess your understanding of oxidation. Web identify the species being oxidised and reduced in each of the following reactions and write their half reactions: The oxidation number of an element.
Oxidation Numbers Worksheet Answers flakeinspire
Web about this quiz & worksheet. Web this product contains two pages. Web the worksheet contains 20 examples that cover all of the oxidation number rules discussed in class. Na2o + h2o g 2 naoh. A pure element has an oxidation number of 0.
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
Na2o + h2o g 2 naoh. I2o5 + h2o g 2 hio 3. Give the oxidation numbers of all the elements in the following. A) li(s) li+(aq) + e−= oxidation b) s(s) + 2e−s2−(aq) = reduction c) cr3+(aq) +. Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a.
The oxidation number of an element in its free (uncombined) state is zero — for example, al(s) or zn(s). Web about this quiz & worksheet. A pure element has an oxidation number of 0. Identify each of the following as examples of oxidation of reduction: Web this product contains two pages. The oxidation number of a monatomic ion equals the. The oxidation number of an element in a monatomic ion equals the charge of the. Give the oxidation numbers of all the elements in the following molecules and ions: Web use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. The handout outlines and illustrates the rules for assigning oxidation numbers. Web the worksheet contains 20 examples that cover all of the oxidation number rules discussed in class. The handout outlines and illustrates the rules for assigning oxidation numbers. Web assign oxidation numbers to each of the atoms in the following compounds: The oxidation number of an element in a. Web instructions on how to use the oxidation number chemistry worksheet. This brief quiz and worksheet will assess your understanding of oxidation. The charge on all free elements is zero. Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. A) li(s) li+(aq) + e−= oxidation b) s(s) + 2e−s2−(aq) = reduction c) cr3+(aq) +. The oxidation number chemistry worksheet contains.
Identify Each Of The Following As Examples Of Oxidation Of Reduction:
Basic oxide of nonacidic cation gives hydroxide in water. Web this product contains two pages. This brief quiz and worksheet will assess your understanding of oxidation. Web this resource can be used for helping students calculate oxidation numbers / oxidation states for the atoms in a.
Oxidation Numbers Hey Name 1.
Amazing activity to practice the oxidation numbers. Web rules for assigning oxidation numbers 1. The oxidation number of an element in its free (uncombined) state is zero — for example, al(s) or zn(s). The oxidation number chemistry worksheet contains.
I2O5 + H2O G 2 Hio 3.
Web the oxidation state of an atom is represented by a positive or negative number called its oxidation number. A pure element has an oxidation number of 0. A pure element has an oxidation number of 0. Web give the oxidation numbers of all the elements in the following molecules and ions:
Web About This Quiz & Worksheet.
The oxidation number of an element in a monatomic ion equals the charge of the. The oxidation number of a monatomic ion equals the. Web the worksheet contains 20 examples that cover all of the oxidation number rules discussed in class. Web instructions on how to use the oxidation number chemistry worksheet.