Worksheet Molarity Answers - Web molarity and molality. Web molarity practice problems #1 1. How many liters of water are needed. 4) 0.5 moles of sodium chloride is dissolved to. A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g /. Web determine the molarity for each of the following solutions: Web results for molarity worksheet 220 + results sort by: To make a 4.00 m solution, how many moles of solute will be. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. You should try to answer the questions without referring to your textbook.
44 Molarity Practice Worksheet Answer Chessmuseum Template Library
Web this quiz and corresponding worksheet will help you gauge your understanding of how to calculate molarity and molality concentration. 4) 0.5 moles of sodium chloride is dissolved to. Web molality = molessolute kilogramssolvent mathematical manipulation of molality is the same as with molarity. To make a 4.00 m solution, how many moles of solute will be needed if 12.0.
Molarity Worksheet 1 worksheet
1) 1 moles of potassium fluoride is dissolved to make 0 l of. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? Web what is the molarity of the following solutions given that: You.
Molarity Pogil Worksheet Answers worksheet
A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g /. You should try to answer the questions without referring to your textbook. A first convert 250 ml to liters, 250/1000 = 0.25 then calculate. To make a 4.00 m solution, how many moles of solute will.
Molarity Worksheet Answer Key
How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Calculate the molarity of each of the following solutions: Web molarity practice worksheet find the molarity of the following solutions: This worksheet can be used in any chemistry class, regardless of the college students’. Web molarity and molality.
7 Molarity Worksheet With Answers /
To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web work in groups on these problems. Web determine the molarity for each of the following solutions: 4) 0.5 moles of sodium chloride is dissolved to. A 3 m aqueous calcium nitrate.
Molarity Worksheet 2 ANSWERS Google Docs
How many liters of water are needed. A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g /. To make a 4.00 m solution, how many moles of solute will be. Web the molarity or molar concentration of a solute is defined as the number of moles.
worksheet. Molarity Worksheet With Answers. Grass Fedjp Worksheet Study
Web molarity practice worksheet find the molarity of the following solutions: Web this quiz and corresponding worksheet will help you gauge your understanding of how to calculate molarity and molality concentration. A 3 m aqueous calcium nitrate. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; 1) 1 moles of potassium fluoride is dissolved to make.
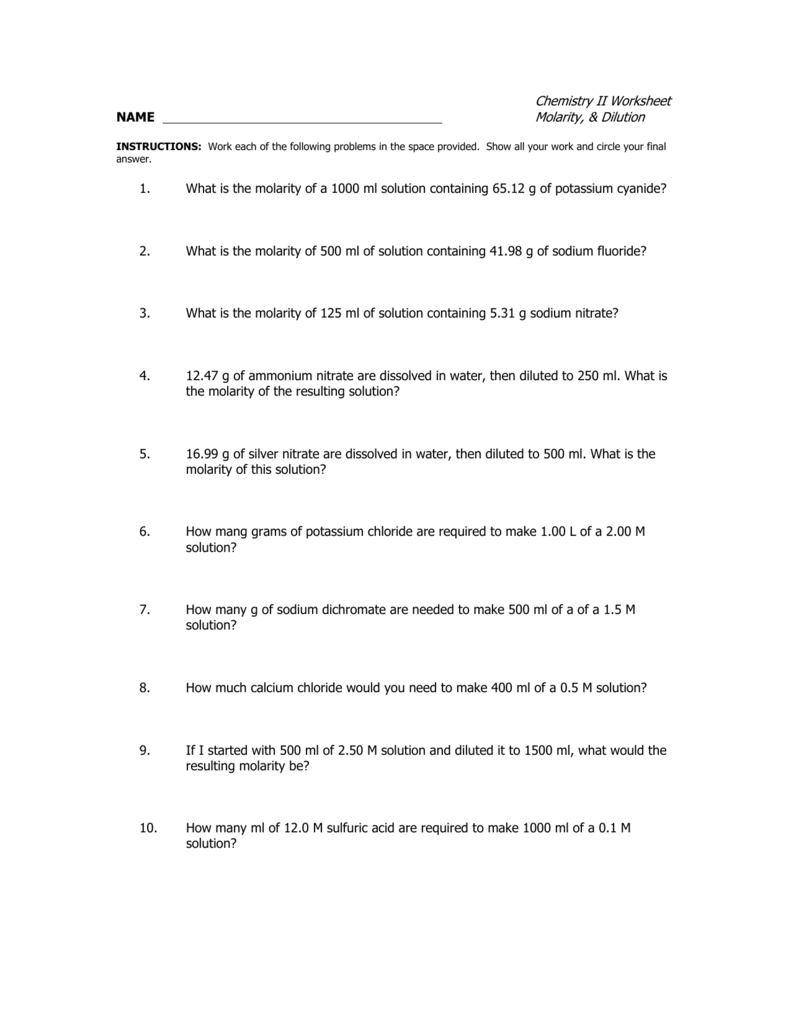
Chemistry II Worksheet NAME Molarity, & Dilution 1. What is the
How many liters of water are needed. To make a 4.00 m solution, how many moles of solute will be. 4) 0.5 moles of sodium chloride is dissolved to. You should try to answer the questions without referring to your textbook. Web this quiz and corresponding worksheet will help you gauge your understanding of how to calculate molarity and molality.
(PDF) Molarity, Molality and Normailty Emmanuel Gyedu Academia.edu
Molarity, molality, normality, and mass percent worksheet ii date _____/_____/_____ period _____ molarity =. Web this quiz and corresponding worksheet will help you gauge your understanding of how to calculate molarity and molality concentration. Calculate the molarity of each of the following solutions: Web results for molarity worksheet 220 + results sort by: To make a 4.00 m solution, how.
50 Molarity Worksheet Answer Key
Web molarity and molality. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web work in groups on these problems. This worksheet can be used in any chemistry class, regardless of the college students’. How many grams of potassium carbonate are needed to make 280 ml of a 2.5.
1) 1 moles of potassium fluoride is dissolved to make 0 l of. 4) 0.5 moles of sodium chloride is dissolved to. Web this customizable and printable worksheet is designed to help students practice calculating the molarity of various solutions. When the solvent is water, we have an aqueous solution. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? A first convert 250 ml to liters, 250/1000 = 0.25 then calculate. You should try to answer the questions without referring to your textbook. Determine the molarity of these solutions: Web the molarity or molar concentration of a solute is defined as the number of moles of solute per liter of solution ( not per liter of. Web molarity and molality. 30 ml of 0.5m h 2 so. How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Web molarity practice worksheet find the molarity of the following solutions: Web molarity is abbreviated as m. Molarity, molality, normality, and mass percent worksheet ii date _____/_____/_____ period _____ molarity =. How many liters of water are needed. Web molality = molessolute kilogramssolvent mathematical manipulation of molality is the same as with molarity. Web work in groups on these problems. Web work in groups on these problems. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are.
30 G Of Co (No 3) 2.6H 2 O In 4.3L Of Solution;
To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. To make a 4.00 m solution, how many moles of solute will be. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric. Web molarity practice worksheet find the molarity of the following solutions:
Show All Work And Circle Your Final Answer.
To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g /. Calculate the molarity of each of the following solutions: 1) 1 moles of potassium fluoride is dissolved to make 0 l of.
How Many Grams Of Potassium Carbonate Are Needed To Make 280 Ml Of A 2.5 M Solution?
Web molarity practice problems #1 1. Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. 4) 0.5 moles of sodium chloride is dissolved to. Web this quiz and corresponding worksheet will help you gauge your understanding of how to calculate molarity and molality concentration.
Web Determine The Molarity For Each Of The Following Solutions:
A first convert 250 ml to liters, 250/1000 = 0.25 then calculate. Web molarity is abbreviated as m. You should try to answer the questions without referring to your textbook. Web what is the molarity of the following solutions given that: