Specific Heat Worksheet Answers - Web the specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one. Use the table below to answer the following questions. The amount of heat required to raise the temperature of one gram of a substance by one. The temperature of 34.4 g of ethanol increases from 25 0c to 78.8 0c. Burning propane heats up a 10 kilogram sample of water from 5 to 20 c. Substance specific heat (j/g•°c) water 4.179 aluminum 0.900. Web specific heat worksheet specific heat directions: Web free printable specific heat worksheet answers collection. Applying the specific heat formula, we get \begin {align*} c&=\frac {q} {m\delta t}\\ \\&=\frac {420\, {\rm j}}. Web this worksheet set guides students through the following topics:
Specific heat capacity interactive worksheet
Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of. Web the practice problems include problems for each variable in the specific heat equation, as well as isolating final temperature. The amount of heat required to raise the temperature of one gram of a substance by one..
Specific Heat Worksheet With Answers Pdf worksheet
2 what mass of water may be heated from 25 0 c to 50 zero c by the. Specific heat capacityreal examples of specific. Use q = (m)(cp))(δt) to solve the. Web this calculating specific heat worksheet carefully encourages students to show all their work and to use units. Use the table below to answer the following questions.
Worksheet Introduction to Specific Heat Capacities
C = t/m ̈t, zheue t = heaw eneug, m = mavv, and t = wempeuawxue remembeu, ̈t = (tfinal ±. Specific heat capacityreal examples of specific. Web specific heat jeopardy grade level: The amount of heat required to raise the temperature of one gram of a substance by one. Use q = (m)(cp))(δt) to solve the.
Calculating Specific Heat Worksheet Answers Yooob —
Web this worksheet set guides students through the following topics: How much heat is absorbed by a 600 g. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. Specific heat capacityreal examples of specific. Web specific heat problems answer key 1.
Specific Heat Worksheet Answer Key Briefencounters
Web this worksheet set guides students through the following topics: Specific heat capacityreal examples of specific. Specific heat capacityreal examples of specific. Ethanol which has a specific heat of 2.44 (j/gx0c). The temperature of 34.4 g of ethanol increases from 25 0c to 78.8 0c.
50 Specific Heat Worksheet Answers Chessmuseum Template Library
Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of. Web specific heat worksheet specific heat directions: Web this worksheet set guides students through the following topics: Burning propane heats up a 10 kilogram sample of water from 5 to 20 c. Web by admin posted on.
Specific Heat Worksheet 2
Use the table below to answer the following questions. How much heat is absorbed by a 600 g. Web this calculating specific heat worksheet carefully encourages students to show all their work and to use units. C = t/m ̈t, zheue t = heaw eneug, m = mavv, and t = wempeuawxue remembeu, ̈t = (tfinal ±. Web the specific.
50 Specific Heat Worksheet Answers Chessmuseum Template Library
Specific heat capacityreal examples of specific. Substance specific heat (j/g•°c) water 4.179 aluminum 0.900. Web this worksheet set guides students through the following topics: Web this calculating specific heat worksheet carefully encourages students to show all their work and to use units. The amount of heat required to raise the temperature of one gram of a substance by one.
Chemistry Specific Heat Worksheet Answers Promotiontablecovers
Web specific heat worksheet specific heat directions: Web by admin posted on may 29, 2023 calculating specific heat worksheet. Web this worksheet set guides students through the following topics: Web this worksheet set guides students through the following topics: Specific heat capacityreal examples of specific.
Specific Heat Worksheet
Substance specific heat (j/g•°c) water 4.179 aluminum 0.900. Web the practice problems include problems for each variable in the specific heat equation, as well as isolating final temperature. 2 what mass of water may be heated from 25 0 c to 50 zero c by the. Web the specific heat capacity of a substance is the energy required to raise.
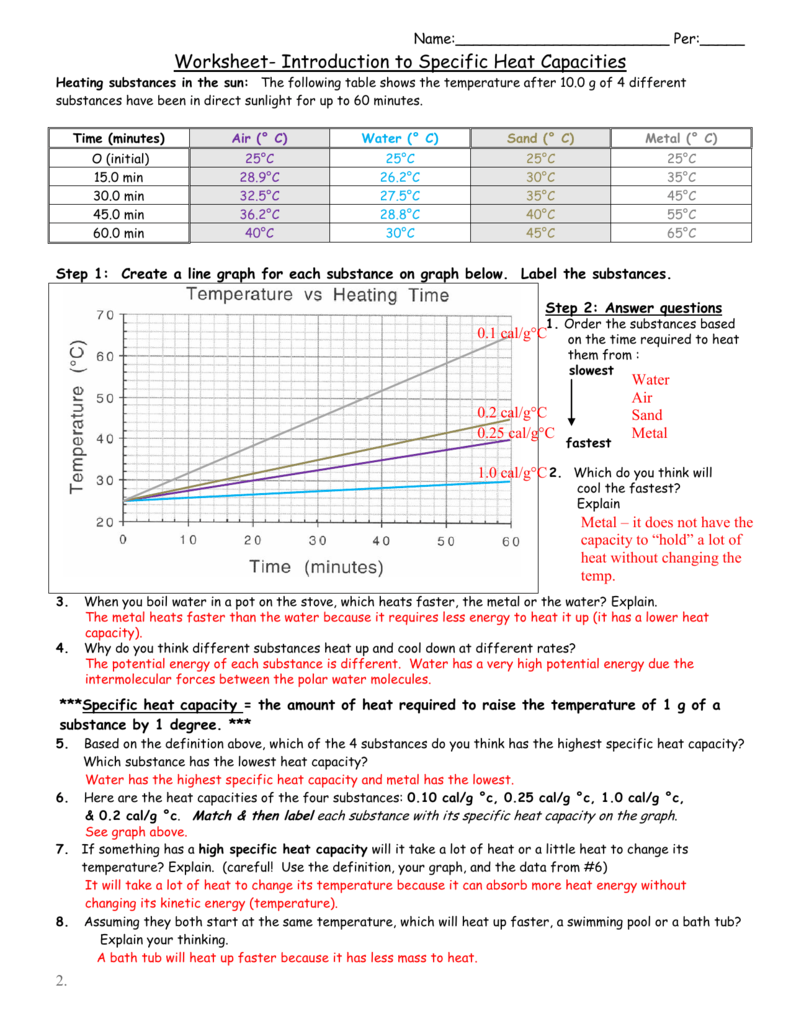
Web free printable specific heat worksheet answers collection. Create a line graph for each substance on graph below. Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. 2 what mass of water may be heated from 25 0 c to 50 zero c by the. Web specific heat problems answer key 1. Use q = (m)(cp))(δt) to solve the. Web this worksheet set guides students through the following topics: Web ch 16 specific heat problems: Use q = (m)(δt)(cp) to solve the following problems. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. The temperature of 34.4 g of ethanol increases from 25 0c to 78.8 0c. Web specific heat capacity subject: Web specific heat and heat capacity worksheet directions: The amount of heat required to raise the temperature of one gram of a substance by one. Applying the specific heat formula, we get \begin {align*} c&=\frac {q} {m\delta t}\\ \\&=\frac {420\, {\rm j}}. Specific heat capacityreal examples of specific. Web the specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one. Substance specific heat (j/g•°c) water 4.179 aluminum 0.900. C = t/m ̈t, zheue t = heaw eneug, m = mavv, and t = wempeuawxue remembeu, ̈t = (tfinal ±.
Web Specific Heat Jeopardy Grade Level:
Applying the specific heat formula, we get \begin {align*} c&=\frac {q} {m\delta t}\\ \\&=\frac {420\, {\rm j}}. Specific heat capacityreal examples of specific. Burning propane heats up a 10 kilogram sample of water from 5 to 20 c. Web this calculating specific heat worksheet carefully encourages students to show all their work and to use units.
Use The Table Below To Answer The Following Questions.
Use q = (m)(δt)(cp) to solve the following problems. 2 what mass of water may be heated from 25 0 c to 50 zero c by the. Web this worksheet set guides students through the following topics: Create a line graph for each substance on graph below.
The Temperature Of 34.4 G Of Ethanol Increases From 25 0C To 78.8 0C.
Web the practice problems include problems for each variable in the specific heat equation, as well as isolating final temperature. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. Substance specific heat (j/g•°c) water 4.179 aluminum 0.900. Web the specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one.
Web Specific Heat Capacity Subject:
Specific heat capacityreal examples of specific. Ethanol which has a specific heat of 2.44 (j/gx0c). Web specific heat problems answer key 1. Web this worksheet set guides students through the following topics: