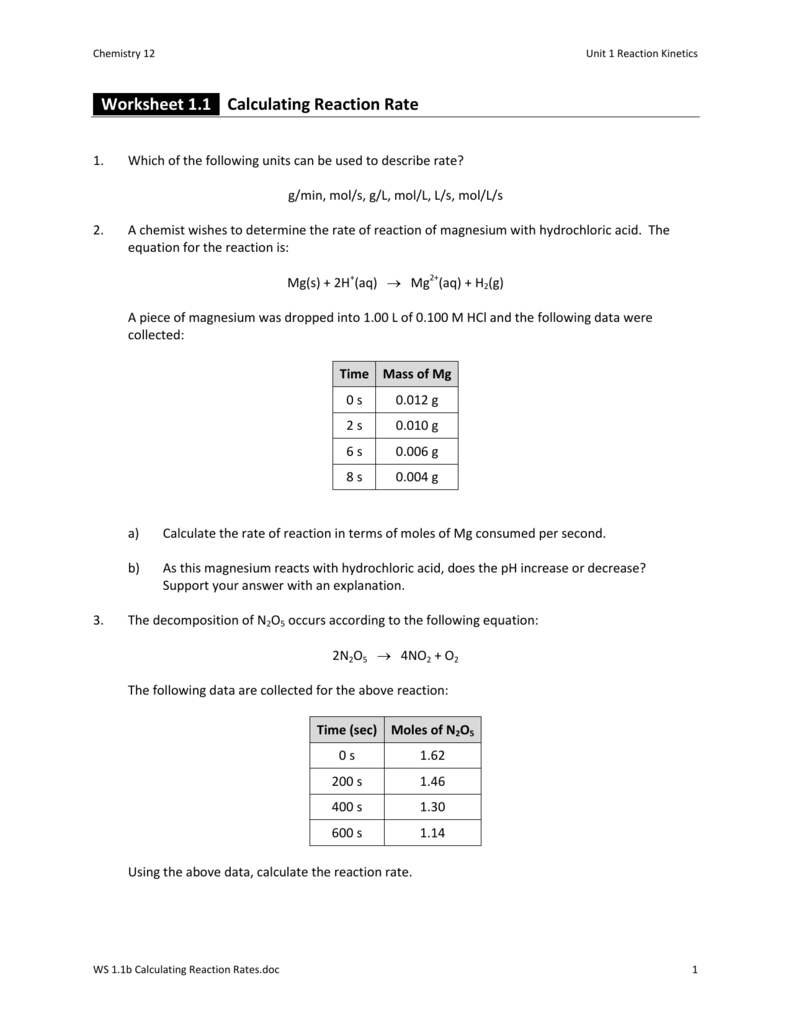
Reaction Rate Worksheet - Web explore what makes a reaction happen by colliding atoms and molecules. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Write the rate expression in terms of δ [reactant]/ δ t and δ [product]/ δ t for the reaction: Web calculate the average reaction times from the combined class data that you will collect together with your teacher. This knowledge can be used to gain insight into the detailed molecular pathway (the mechanism) by which the reaction occurs. The equation for the reaction is: Use your graph to determine the instantaneous reaction rate at 250 ms. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work,. Reaction rate refers to how quickly or slowly the _____ disappear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid.
Rates Of Reaction Worksheet —
Some of the worksheets for this concept are rate of reaction work, work. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products, too. The equation for the reaction is: Given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant.
Rates of Reaction Worksheet 7.2
Rates of reactions practice means progress boost your grades with free daily practice questions. Use your graph to determine the instantaneous reaction rate at 250 ms. Web relative rates of reaction. Write the rate expression in terms of δ [reactant]/ δ t and δ [product]/ δ t for the reaction: This knowledge can be used to gain insight into the.
13 Best Images of Worksheet Reaction Rates Answer Worksheet Measuring
Use your graph to determine the instantaneous reaction rate at 250 ms. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Web determine the average rate for the reaction between each data point. The rate of a reaction may be expressed in terms of the change.
Reaction Rates worksheet from EdPlace
Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work,. Reaction rate refers to how quickly or slowly the _____ disappear. N 2 + 3 h 2 → 2 n h 3 2. Web calculating rates of reaction: Rates of reactions practice means progress boost your grades with free.
Factors Affecting the Rate of Reaction SPM Chemistry
A reaction has the experimental rate law,. Web determine the average rate for the reaction between each data point. Some of the worksheets for this concept are rate of reaction work, work. Use your graph to determine the instantaneous reaction rate. The rate of a reaction may be expressed in terms of the change in the amount of any reactant.
Worksheet for Rates of Reaction Unit DocsLib
This knowledge can be used to gain insight into the detailed molecular pathway (the mechanism) by which the reaction occurs. The rate of a reaction may be expressed in terms of the change in the amount of any reactant or. Web determine the average rate for the reaction between each data point. The equation for the reaction is: Web worksheets.
Ms J's Chemistry Class 10/01/2016 11/01/2016
Web in the units on thermochemistry and chemical kinetics, you will learn how energy is absorbed and given off during chemical. A study of reaction _____ is called chemical _____. Some of the worksheets for this concept are rate of reaction work, work. Design experiments with different reactions,. Reaction rate refers to how quickly or slowly the _____ disappear.
Method of Initial Rate Worksheet
Rates of reactions practice means progress boost your grades with free daily practice questions. Reaction rate refers to how quickly or slowly the _____ disappear. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for.
13 Best Images of Worksheet Reaction Rates Answer Worksheet Measuring
Web relative rates of reaction. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for each set of data points Use your graph to determine the.
Worksheet 1.1 Calculating Reaction Rate
Web explore what makes a reaction happen by colliding atoms and molecules. Reaction rate refers to how quickly or slowly the _____ disappear. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products, too. Web relative rates of reaction. Web in this practice worksheet students will.
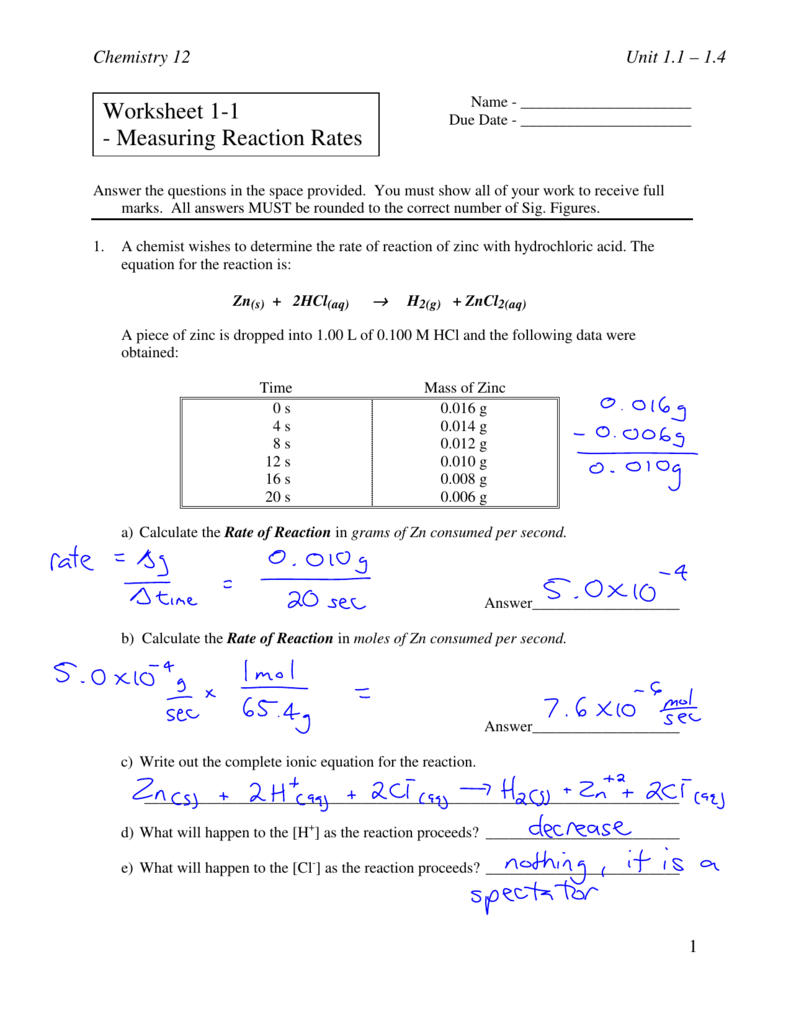
N 2 + 3 h 2 → 2 n h 3 2. The rate of a reaction may be expressed in terms of the change in the amount of any reactant or. Write the rate expression in terms of δ [reactant]/ δ t and δ [product]/ δ t for the reaction: Some of the worksheets for this concept are rate of reaction work, work. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. Reaction rate refers to how quickly or slowly the _____ disappear. This knowledge can be used to gain insight into the detailed molecular pathway (the mechanism) by which the reaction occurs. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work,. Rates of reactions practice means progress boost your grades with free daily practice questions. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products, too. Use your graph to determine the instantaneous reaction rate. Given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for each set of data points Web determine the average rate for the reaction between each data point. Design experiments with different reactions,. Web explore what makes a reaction happen by colliding atoms and molecules. Web relative rates of reaction. A study of reaction _____ is called chemical _____. Web determine the average rate for the reaction between each data point. Web in the units on thermochemistry and chemical kinetics, you will learn how energy is absorbed and given off during chemical. Web calculating rates of reaction:
Web Determine The Average Rate For The Reaction Between Each Data Point.
Web calculate the average reaction times from the combined class data that you will collect together with your teacher. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. The rate of a reaction may be expressed in terms of the change in the amount of any reactant or. Web determine the average rate for the reaction between each data point.
Write The Rate Expression In Terms Of Δ [Reactant]/ Δ T And Δ [Product]/ Δ T For The Reaction:
Rates of reactions practice means progress boost your grades with free daily practice questions. Design experiments with different reactions,. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The equation for the reaction is:
Web In The Units On Thermochemistry And Chemical Kinetics, You Will Learn How Energy Is Absorbed And Given Off During Chemical.
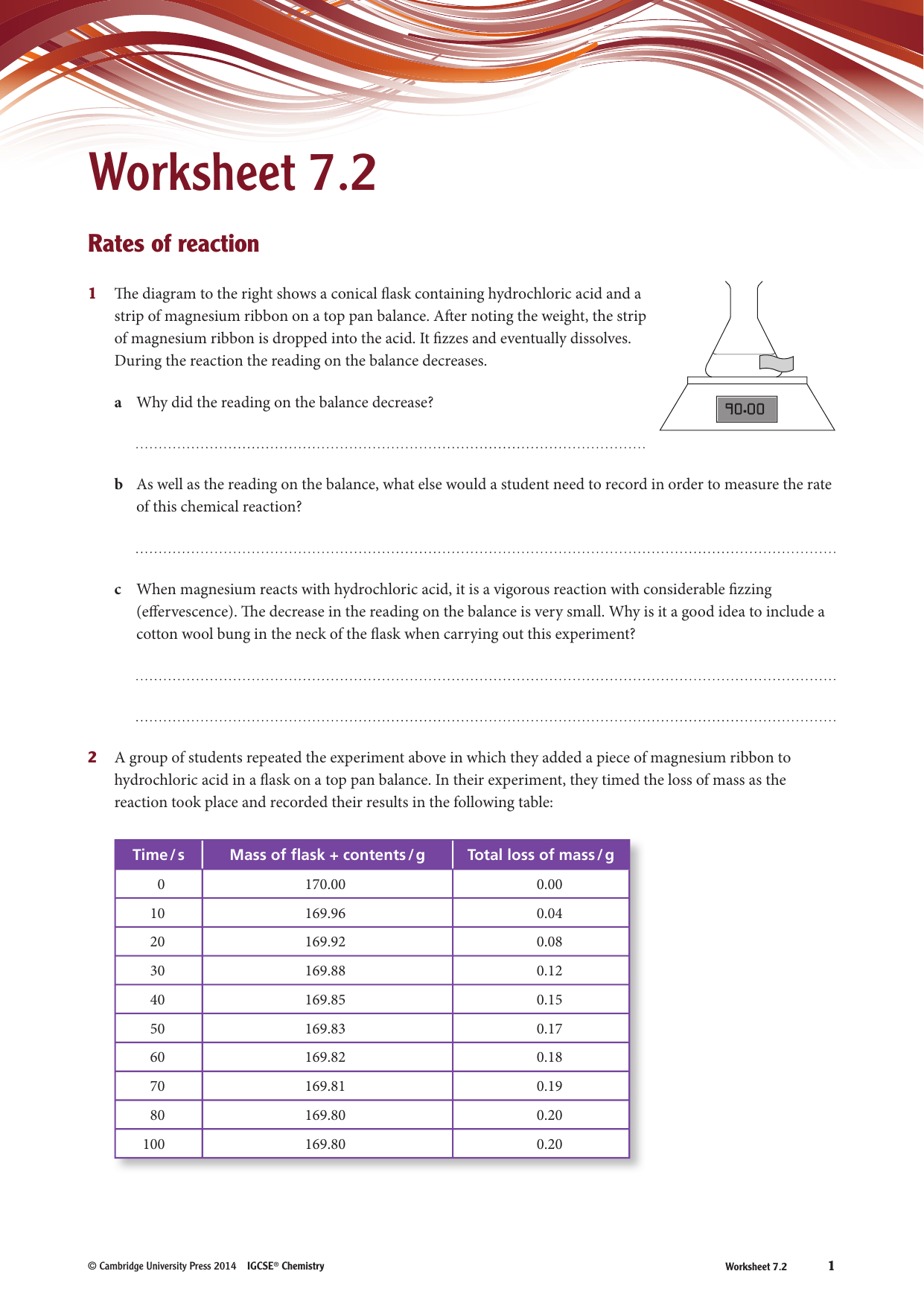
Reaction rate refers to how quickly or slowly the _____ disappear. Web the rate of the reaction is determined by measuring the volume of h x 2 (g) \ce{h2}(g) h x 2 (g) produced every five seconds. N 2 + 3 h 2 → 2 n h 3 2. This knowledge can be used to gain insight into the detailed molecular pathway (the mechanism) by which the reaction occurs.
Web Experimentally Determined Rate Law Expressions Show How The Rate Of A Reaction Depends Upon The Concentrations Of The Reactants And Sometimes The Products, Too.
Use your graph to determine the instantaneous reaction rate at 250 ms. Web explore what makes a reaction happen by colliding atoms and molecules. Given that the reaction is first order in \(no\) and in \(o_3\), determine the rate constant using your calculated rate for each set of data points Web relative rates of reaction.


.jpg)