Rate Law Worksheet With Answers - A reaction has the experimental rate law, rate = k[a]2. Web a) rate is increased by a factor of 9 (nine times as fast as the original rate) b). Web a) rate = k [no2]2 b) rate = k c) rate = k [h2] [br2]1/2 d) rate = k [no]2[o a) second order b) zero order c) 3/2 order d) third. Web reaction order and rate law expression worksheet. Web using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that. Web the rate expression or rate law expression is the relation between reaction rate and the concentrations of reactants given by a. The method of initial rates can be used to establish the order of reaction and give an estimate of the rate. Web calculate the new rate of reaction if the concentration of hi is 0.0885 m.7 m / s r = 5 × 10 −4 6) the decomposition of n 2 o 5. Web 1) concentration of reactants: Reaction rate increases as concentration of reactants increases because number of collisions.
Rate Law Worksheet With Answers
Web initial rates problems key 1. A reaction has the experimental rate law, rate = k[a]2. Web chemistry questions and answers; Web a) rate is increased by a factor of 9 (nine times as fast as the original rate) b). What is the reaction order in terms of \(h^+\)?
Rate Law Worksheet With Answers
N02(g) + 02(g) 2n205(g) kc.o¿shx.011hj initial rate 3.1 x mol/l.s. Topics to know for the quiz include. What is the reaction order in terms of \(h^+\)? Given reaction rate data for: What is the overall reaction order of this reaction?
Rate Law Worksheet With Answers
If rate 1 =k[a]2, then rate 2. Reaction rate increases as concentration of reactants increases because number of collisions. Web using calculus, the differential rate law for a chemical reaction can be integrated with respect to time to give an equation that. Given reaction rate data for: If the volume occupied by the reacting.
Rate Law Worksheet.doc
Web chemistry questions and answers; N02(g) + 02(g) 2n205(g) kc.o¿shx.011hj initial rate 3.1 x mol/l.s. Given the following equations and experimental data, write the correct. Given reaction rate data for: Web rate law worksheet 1.
AP Chemistry Page
For a reaction where the rate equation is r = k[nh 4 (aq)][no 2 (aq)], +. Web this quiz and worksheet will help gauge your understanding of rate constant and rate laws. A reaction has the experimental rate law, rate = k[a]2. What is the reaction order in terms of \(h^+\)? If the volume occupied by the reacting.
Principles of Chem 2
Web rate law worksheet 1. A reaction has the experimental rate law, rate = k[a]2. How will the rate change if the concentration of a is tripled? If rate 1 =k[a]2, then rate 2. Web use the following data to answer the questions below:
Rate Law Worksheet With Answers
Given the following equations and experimental data, write the correct. The method of initial rates can be used to establish the order of reaction and give an estimate of the rate. Determine the order of each. For a reaction where the rate equation is r = k[nh 4 (aq)][no 2 (aq)], +. How will the rate change if the concentration.
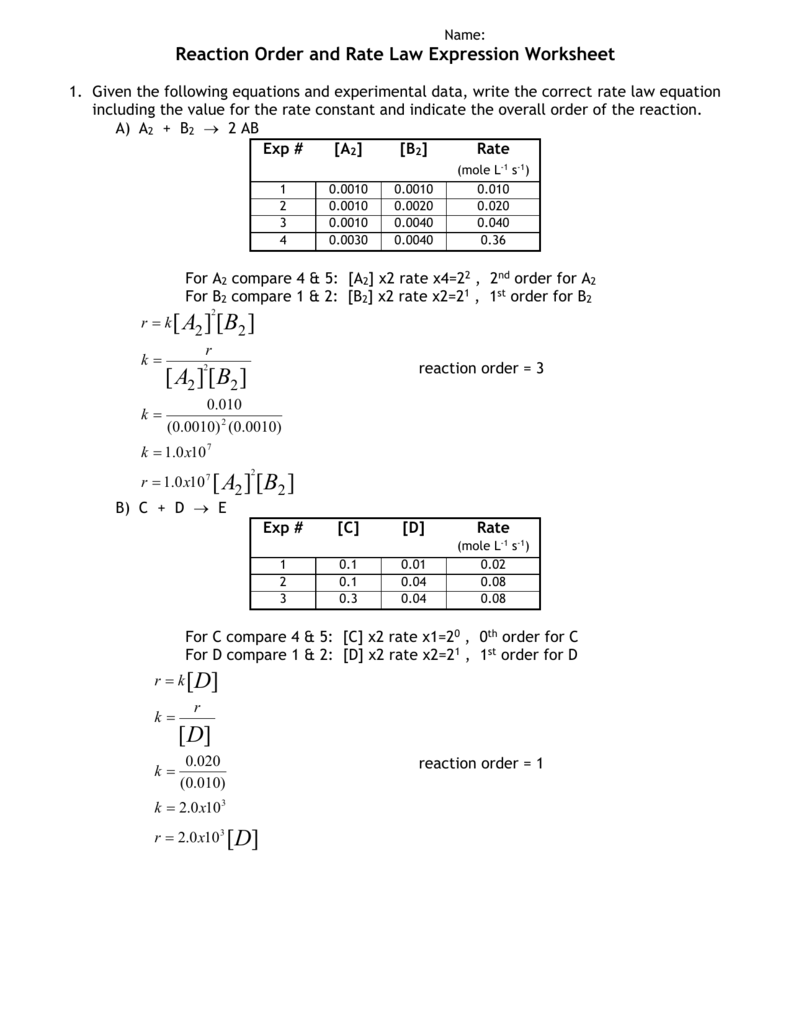
Reaction Order and Rate Law Expression Worksheet
What is the reaction order in terms of \(h^+\)? The rate of a reaction is given by k [a][b]. Reaction rate increases as concentration of reactants increases because number of collisions. A reaction has the experimental rate law, rate = k[a]2. Web calculate the new rate of reaction if the concentration of hi is 0.0885 m.7 m / s r.
Rate Law Worksheet 2
Web chemistry questions and answers; Web this quiz and worksheet will help gauge your understanding of rate constant and rate laws. How will the rate change if the concentration of a is tripled? Determine the order of each. Topics to know for the quiz include.
Rate Laws Presentation Chemistry
N02(g) + 02(g) 2n205(g) kc.o¿shx.011hj initial rate 3.1 x mol/l.s. Web initial rates problems key 1. Web reaction order and rate law expression worksheet. What is the overall reaction order of this reaction? How will the rate change if the concentration of a is tripled?
Given reaction rate data for: Given the following equations and experimental data, write the correct. N02(g) + 02(g) 2n205(g) kc.o¿shx.011hj initial rate 3.1 x mol/l.s. Determine the order of each. For a reaction where the rate equation is r = k[nh 4 (aq)][no 2 (aq)], +. If the volume occupied by the reacting. Web rate laws (sometimes called differential rate laws) or rate equations are mathematical expressions that describe the relationship. Web chemistry questions and answers; Web use the following data to answer the questions below: Reaction rate increases as concentration of reactants increases because number of collisions. What is the reaction order in terms of \(h^+\)? Web 1) concentration of reactants: What is the overall reaction order of this reaction? If rate 1 =k[a]2, then rate 2. Topics to know for the quiz include. The rate of a reaction is given by k [a][b]. Web this quiz and worksheet will help gauge your understanding of rate constant and rate laws. Web calculate the new rate of reaction if the concentration of hi is 0.0885 m.7 m / s r = 5 × 10 −4 6) the decomposition of n 2 o 5. Web initial rates problems key 1. Web a) rate is increased by a factor of 9 (nine times as fast as the original rate) b).
What Is The Reaction Order In Terms Of \(H^+\)?
Web this quiz and worksheet will help gauge your understanding of rate constant and rate laws. Determine the order of each. Web a) rate = k [no2]2 b) rate = k c) rate = k [h2] [br2]1/2 d) rate = k [no]2[o a) second order b) zero order c) 3/2 order d) third. The rate of a reaction is given by k [a][b].
Web Use The Following Data To Answer The Questions Below:
Web calculate the new rate of reaction if the concentration of hi is 0.0885 m.7 m / s r = 5 × 10 −4 6) the decomposition of n 2 o 5. Given the following equations and experimental data, write the correct. Web rate law worksheet 1. Web chemistry questions and answers;
Web Using Calculus, The Differential Rate Law For A Chemical Reaction Can Be Integrated With Respect To Time To Give An Equation That.
The method of initial rates can be used to establish the order of reaction and give an estimate of the rate. How will the rate change if the concentration of a is tripled? Web rate laws (sometimes called differential rate laws) or rate equations are mathematical expressions that describe the relationship. If the volume occupied by the reacting.
Chem 1120 Name Integrated Rate Laws Worksheet 2) The Second Order Reaction:
Web reaction order and rate law expression worksheet. Web a) rate is increased by a factor of 9 (nine times as fast as the original rate) b). Web initial rates problems key 1. Web the rate expression or rate law expression is the relation between reaction rate and the concentrations of reactants given by a.








.PNG)