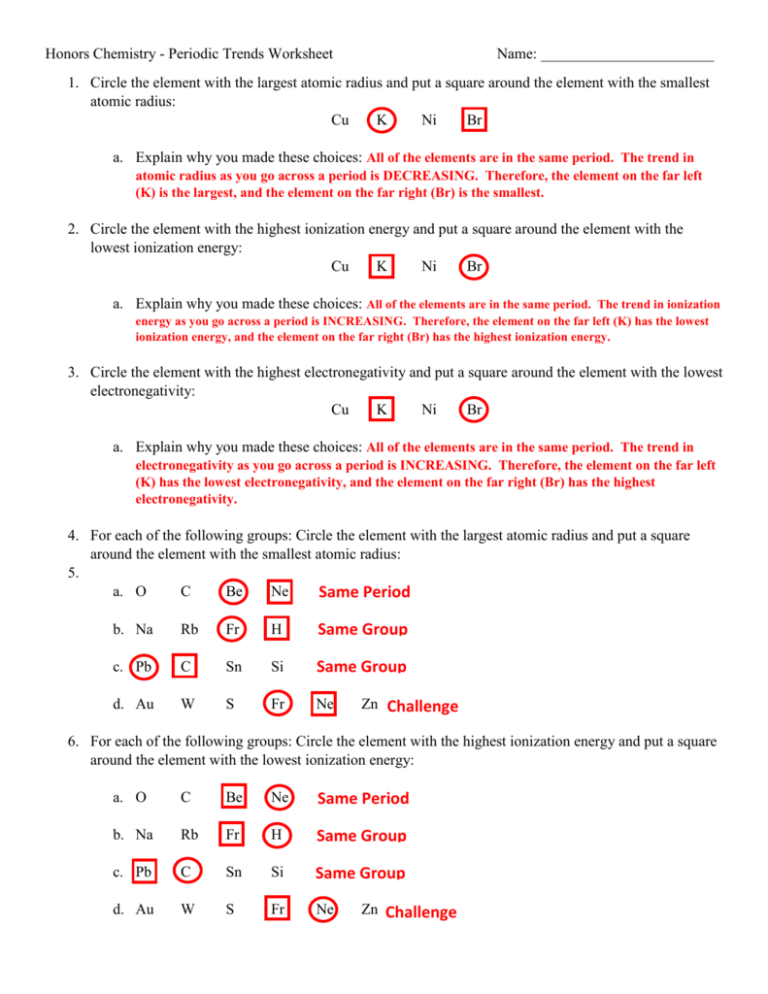
Periodic Trends Ionization Energy Worksheet Answers - Web another property of atoms is called the ionization energy. Understand the reasons for metallic, nonmetallic, and. Pb c sn si same group d. Web know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. The first four ionization energies for element x (not in kj/mol) are 170, 350, 1800, 2500. It increases as you go across a period. The more strongly the electron is. Sulfur, oxygen, neon, aluminium it is higher up on the periodic. The number of protons found in an. O c be ne same period b.
Chemistry 128 > Speltz > Flashcards > CHE 128 Exam 3 StudyBlue
Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. Select the element with the highest ionization energy name date a f b. Web another property of atoms is called the ionization energy. Web specific patterns of certain elemental characteristics are present in the periodic table. Web a) there is a general trend in ionization energy across the.
Periodic Trends Worksheet Answers
Web indicate whether the following properties increase or decrease from left to right across the periodic table. Web this worksheet has 42 multiple choice questions in the following two topics of. The first four ionization energies for element x (not in kj/mol) are 170, 350, 1800, 2500. Web rank the following elements by increasing electronegativity: The amount of energy needed.
Periodic Trends Worksheet 1 answers
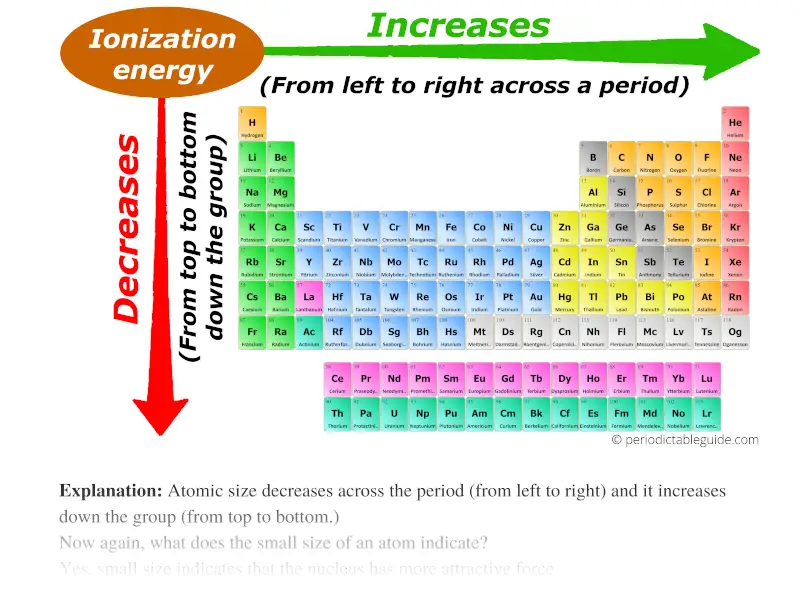
Web major periodic trends include: Periodic trends worksheet for each pair of elements, use the general periodic trends given in class and in the. Web ionization energy for each of the following sets of atoms, rank them from lowest to highest ionization energy. Mg, si, s mg, ca, ba f, cl, br ba, cu, ne si, p,. Web this worksheet.
35 Chemistry Chapter 6 The Periodic Table Worksheet Answers support
Sulfur, oxygen, neon, aluminium it is higher up on the periodic. Web specific patterns of certain elemental characteristics are present in the periodic table. Select the element with the highest ionization energy name date a f b. Pb c sn si same group d. O c be ne same period b.
The Parts of the Periodic Table
Web specific patterns of certain elemental characteristics are present in the periodic table. C, n, si (b) largest radius: Understand the reasons for metallic, nonmetallic, and. The number of protons found in an. Worksheet chapter 6.1 periodic trends 1.
Periodic Trends in Ionization Energy CK12 Foundation
Mg, si, s mg, ca, ba f, cl, br ba, cu, ne si, p,. Web circle the best choice in each list: Au w s fr ne zn challenge 6. Web major periodic trends include: Sulfur, oxygen, neon, aluminium it is higher up on the periodic.
What Is Ionization Energy? Definition and Trend
Sulfur, oxygen, neon, aluminium it is higher up on the periodic. Mg, si, s mg, ca, ba f, cl, br ba, cu, ne si, p,. Worksheet chapter 6.1 periodic trends 1. Web a) there is a general trend in ionization energy across the periodic table; Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character.
All Periodic Trends in Periodic Table (Explained with Image)
Worksheet chapter 6.1 periodic trends 1. Web explains ionization energy, trends in ionization energy, and electron shielding. Web rank the following elements by increasing electronegativity: Web major periodic trends include: Sulfur, oxygen, neon, aluminium it is higher up on the periodic.
Periodic Behavior Presentation Chemistry
Web circle the best choice in each list: Web know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. The first four ionization energies for element x (not in kj/mol) are 170, 350, 1800, 2500. The more strongly the electron is. Pb c sn si same group d.
Atomic Radius, ionization Energy, periodic Trends, electronegativity
Au w s fr ne zn challenge 6. Choose the element with the greatest first ionization energy:. Web a) there is a general trend in ionization energy across the periodic table; Web this worksheet has 42 multiple choice questions in the following two topics of. It increases as you go across a period.
Worksheet chapter 6.1 periodic trends 1. Choose the element with the greatest first ionization energy:. The amount of energy needed to remove one valence electron from an atom. Web rank the following elements by increasing electronegativity: Periodic trends worksheet for each pair of elements, use the general periodic trends given in class and in the. Web indicate whether the following properties increase or decrease from left to right across the periodic table. Pb c sn si same group d. It increases as you go across a period. Web this worksheet has 42 multiple choice questions in the following two topics of. Mg, si, s mg, ca, ba f, cl, br ba, cu, ne si, p,. Na rb fr h same group c. Web explains ionization energy, trends in ionization energy, and electron shielding. Web in this simulation, students can investigate the periodic trends of atomic radius, ionization energy, and ionic radius. The first four ionization energies for. Le groupe energyzon à travers ses différentes filiales est. Web ionization energy for each of the following sets of atoms, rank them from lowest to highest ionization energy. O c be ne same period b. Understand the reasons for metallic, nonmetallic, and. Web know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. B) there is a general trend in.
Web In This Simulation, Students Can Investigate The Periodic Trends Of Atomic Radius, Ionization Energy, And Ionic Radius.
The more strongly the electron is. Sulfur, oxygen, neon, aluminium it is higher up on the periodic. O c be ne same period b. Mg, si, s mg, ca, ba f, cl, br ba, cu, ne si, p,.
Pb C Sn Si Same Group D.
What is the atomic number? Web circle the best choice in each list: It increases as you go across a period. Web specific patterns of certain elemental characteristics are present in the periodic table.
Na Rb Fr H Same Group C.
(a) highest first ionization energy: The first four ionization energies for. Web indicate whether the following properties increase or decrease from left to right across the periodic table. Web a) there is a general trend in ionization energy across the periodic table;
The Number Of Protons Found In An.
Au w s fr ne zn challenge 6. The amount of energy needed to remove one valence electron from an atom. Web another property of atoms is called the ionization energy. Electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character.







.PNG)
