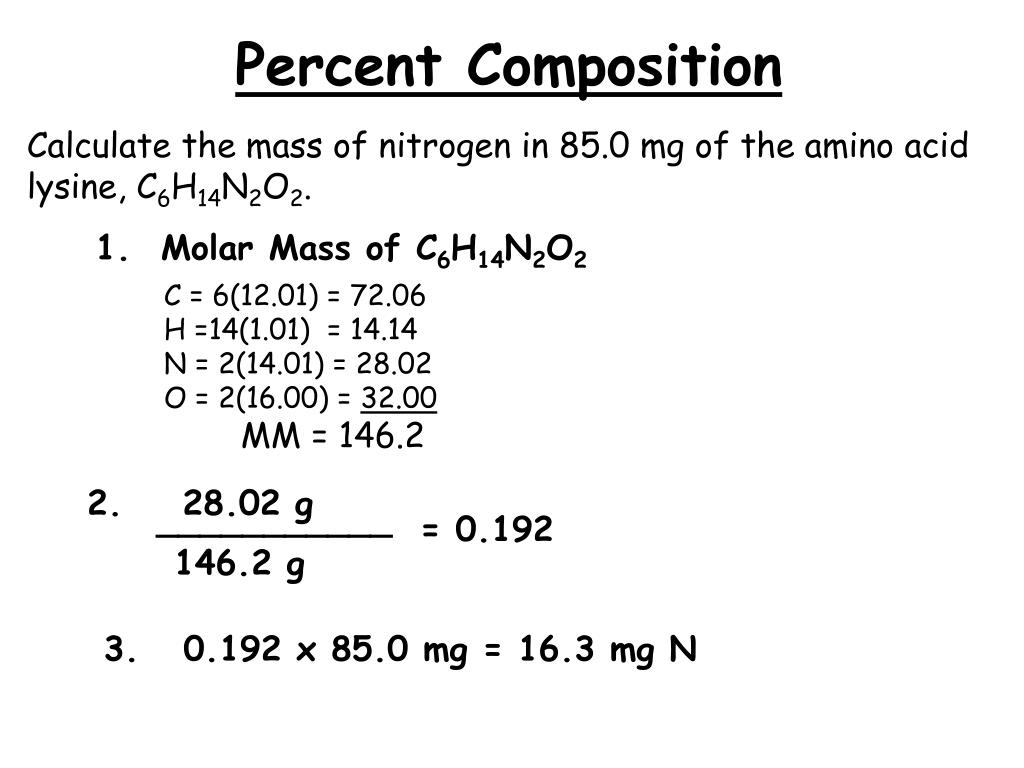Percent Composition And Molecular Formula Worksheet - What's the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Web the percent composition of a compound can also be determined from the formula of the compound. %h = 1.85gh 12.04gcompound × 100% = 15.4%. Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web percent composition and molecular formula worksheet. (a) p 4 (b) h 2 o (c) ca(no 3) 2 (d) ch 3 co 2 h. What’s the empirical formula of a molecule containing 65.5%. Web percent composition and molecular formula worksheet key 1.
Percent Composition Empirical And Molecular Formulas Worksheet Answers
Percentage composition and empirical formulas. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of. To calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of.
PPT Percent Composition, Empirical Formulas, Molecular Formulas
%h = 1.85gh 12.04gcompound × 100% = 15.4%. Web the molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and. %c = 7.34gc 12.04gcompound × 100% = 61.0%. Calculate the percent of nitrogen in urea, nh2conh2. Web the percent composition of a compound can also be determined from the formula of the compound.
Molecular Mass and Percent Composition Worksheet Worksheet for 9th
Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. Web what is the molecular formula? Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Calculate the percent of nitrogen in urea, nh2conh2. To calculate percent composition, we divide.
Molecular Mass and Percent Composition Worksheet for 9th 12th Grade
Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. Recall, percent is parts per hundred. What's the empirical formula of a molecule containing 65.5% carbon, 5.5%. Join to access all included. Web percent composition and molecular formula worksheet key.
percent composition and molecular formula worksheet key
Web percent composition and molecular formula worksheet key. %c = 7.34gc 12.04gcompound × 100% = 61.0%. Calculate the mass percent of carbon, nitrogen and oxygen in acetamide, c2h5no. Web calculate the molecular or formula mass of each of the following: Calculate the percent of nitrogen in urea, nh2conh2.
Percent Composition Chemistry Worksheet Comp Emp Molec Formula Ms
%n = 2.85gn 12.04gcompound × 100% = 23.7%. (a) p 4 (b) h 2 o (c) ca(no 3) 2 (d) ch 3 co 2 h. Web the molecular formula of chloroform indicates that a single molecule contains one carbon atom, one hydrogen atom, and. Web percent composition and molecular formula worksheet 1. Determine the empirical formulas for compounds with the.
Percent Composition and Molecular Formula Worksheet YouTube
(a) p 4 (b) h 2 o (c) ca(no 3) 2 (d) ch 3 co 2 h. Calculate the mass percent of carbon, nitrogen and oxygen in acetamide, c2h5no. Join to access all included. To calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to a percentage:.
Mr. Zehner's Chemistry Class February 2011
Web percent composition and molecular formula worksheet what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web calculate the molecular or formula mass of each of the following: Web percent composition and molecular formula worksheet. Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. What’s the empirical formula.
Percent Composition, Empirical & Molecular Formula YouTube
Find the molar mass of all the elements in the compound in grams per mole. What's the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web percent composition and molecular formula worksheet what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. To calculate percent composition, we divide the experimentally derived mass of each element by the overall.
Percent Composition
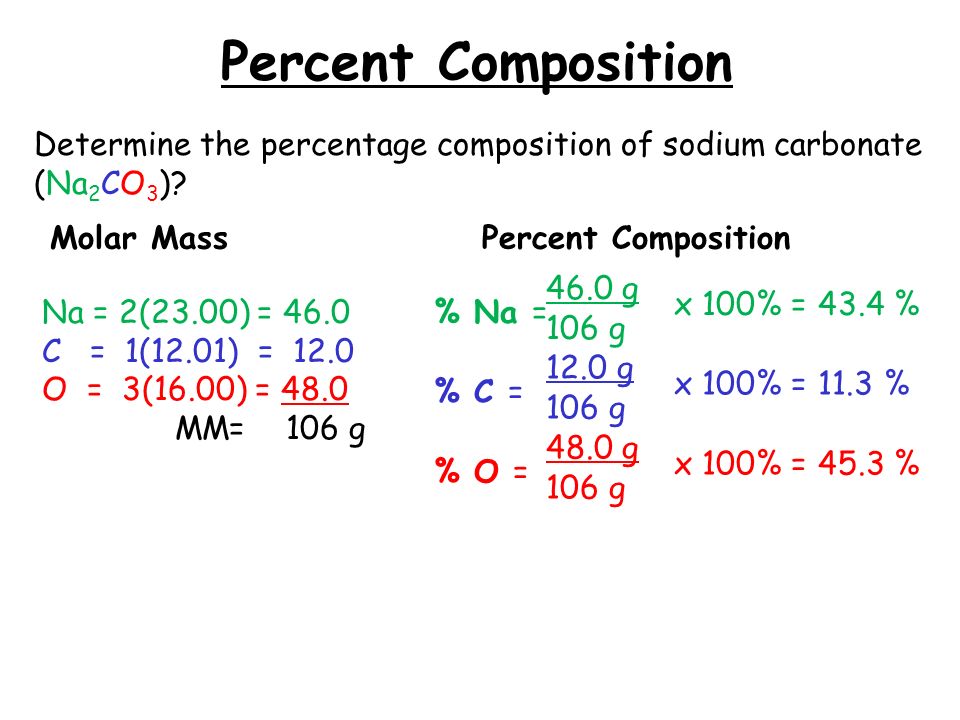
Web what is the molecular formula? Percentage composition and empirical formulas. Web the percent composition of a compound is the percent, by mass, of each individual element within the compound. What’s the empirical formula of a molecule containing 65.5%. Web the percent composition of a compound can also be determined from the formula of the compound.
Web percent composition and molecular formula worksheet what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. What’s the empirical formula of a molecule containing 65.5% carbon,. Web calculate the molecular or formula mass of each of the following: Determine the empirical formulas for compounds with the following percent compositions: Web percent composition and molecular formula worksheet key. What's the empirical formula of a molecule containing 65.5% carbon, 5.5%. Find the molar mass of all the elements in the compound in grams per mole. Web percent composition and molecular formula worksheet. Web percent composition and molecular formula worksheet key 1. Join to access all included. Find the molecular mass of the entire compound. %n = 2.85gn 12.04gcompound × 100% = 23.7%. Web what is the molecular formula? Web percent composition and molecular formula worksheet 1. Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. To calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to a percentage: Web the percent composition of a compound is the percent, by mass, of each individual element within the compound. Calculate the percent of nitrogen in urea, nh2conh2. (a) p 4 (b) h 2 o (c) ca(no 3) 2 (d) ch 3 co 2 h.
Web Guided Worksheet That Reviews Determining Percent Composition Of A Compound, Finding Hydrate Formulas Based On Mass From A.
%h = 1.85gh 12.04gcompound × 100% = 15.4%. Join to access all included. Web the percent composition of a compound is the percent, by mass, of each individual element within the compound. Recall, percent is parts per hundred.
Web Percent Composition And Molecular Formula Worksheet Key 1.
Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5%. Web the percent composition of a compound can also be determined from the formula of the compound. Web what is the molecular formula? Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole.
Web Percent Composition And Molecular Formula Worksheet 1.
To calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to a percentage: %c = 7.34gc 12.04gcompound × 100% = 61.0%. Web calculate the molecular or formula mass of each of the following: What's the empirical formula of a molecule containing 65.5% carbon, 5.5%.
Calculate The Percent Of Nitrogen In Urea, Nh2Conh2.
Web we can use this information to find the percent composition of the compound. Find the molecular mass of the entire compound. Web this molar mass and percentage composition worksheet also includes: What’s the empirical formula of a molecule containing 65.5%.