Partial Pressures Of Gas Chem Worksheet 14-6 Answer Key - = (0.76)(2.3 atm) = 1.75 atm p #! 5.51 atm 507.1 kpa 812 mm hg 2.41 l key volume 1.23 dm3) 2.82 atm of pressure. Consider an ice cube made of 8 water molecules in an absolutely empty. What would be the partial pressure of each of the gases in a container at 50 °c in which there is 0.20 mole n2 and 0.10 mole co2. Ideal gas law fact and conceptual questions; Solve each of the following problems. Container c (with volume 1.42 dm3) into container d (with volume. What is the partial pressure of each gas in. The mole fraction of each gas d. What would be the partial pressure of each of the gases in a.
Solved Worksheet 15 (Gen Chem) Chemical Equilibrium 10.
Web in terms of partial pressures: Relating temperature, volume, and pressure;. A container containing 5.00 l of a gas. Solve each of the following problems. The total pressure of the mixture e.
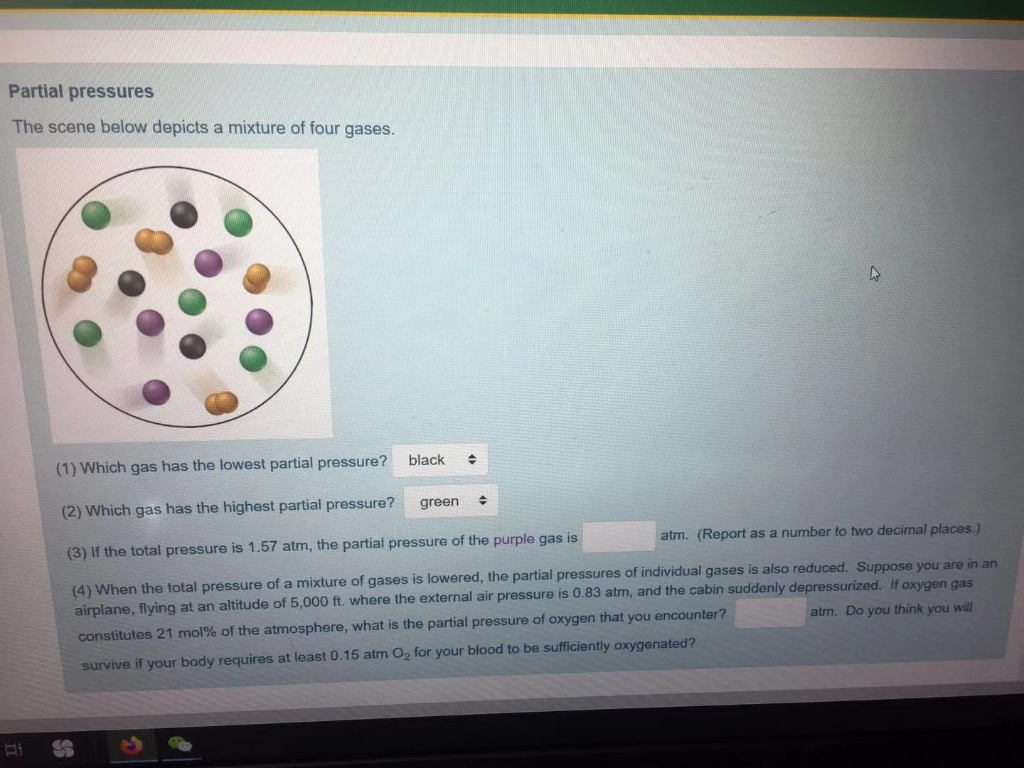
Solved Partial pressures The scene below depicts a mixture
Calculate the decrease in temperature when 6.00 l at 20.0 °c is compressed to 4.00 l. Web the partial pressure of each gas is 0.265 atm. Relating temperature, volume, and pressure;. Web what is the total pressure of the gas? What would be the partial pressure of each of the gases in a container at 50 °c in which there.
Equilibrium And Pressure Gizmo Answer Key Solved Activity B The
Solve each of the following problems. 5.51 atm 507.1 kpa 812 mm hg 2.41 l key volume 1.23 dm3) 2.82 atm of pressure. Web as the pressure increases,. Web a gas mixture contains oxygen, argon and nitrogen. Calculate the decrease in temperature when 6.00 l at 20.0 °c is compressed to 4.00 l.
Dalton S Law Of Partial Pressures Worksheet worksheet
The mole fraction of each gas d. Solve each of the following problems. Web the partial pressure of a gas can be calculated using the ideal gas law, which we will cover. Web in terms of partial pressures: Web a sample of gas.
10.6 Gas Mixtures and Partial Pressures Chemistry LibreTexts
Web in terms of partial pressures: The partial pressure of each gas 4. What would be the partial pressure of each of the gases in a. Web a gas mixture contains oxygen, argon and nitrogen. Web we refer to the pressure.
Partial Pressures Of Gas Chem Worksheet 146 Answer Key
Relating temperature, volume, and pressure;. The total pressure of the mixture e. Web a sample of gas. Web we refer to the pressure. = (0.045)(2.3 atm) = 0.10 atm p !
gas laws Archives HVAC School
Dalton’s law of partial pressure. Web the partial pressure of each gas is 0.265 atm. The partial pressure of the. Relating temperature, volume, and pressure;. What is the partial pressure of each gas in.
Gas pressure worksheet
Web what is the partial pressure of each of the gases? Web in terms of partial pressures: 5.51 atm 507.1 kpa 812 mm hg 2.41 l key volume 1.23 dm3) 2.82 atm of pressure. Web a gas mixture contains oxygen, argon and nitrogen. The mole fraction of each gas d.
Dalton S Law Of Partial Pressures Worksheet worksheet
Ton's law of partial pressures states that the total pressure exerted. Web what is the partial pressure of each of the gases? The mole fraction of each gas d. Web in terms of partial pressures: What would be the partial pressure of each of the gases in a container at 50 °c in which there is 0.20 mole n2 and.
CHEM 120 Textbook Notes Winter 2017, Chapter 4 Gases Partial
What is the partial pressure of each gas in. Ideal gas law fact and conceptual questions; Consider an ice cube made of 8 water molecules in an absolutely empty. What are the mole fractions of all the gases in the mixture? Calculate the decrease in temperature when 6.00 l at 20.0 °c is compressed to 4.00 l.
What would be the partial pressure of each of the gases in a container at 50 °c in which there is 0.20 mole n2 and 0.10 mole co2. The mole fraction of each gas d. The total pressure of the mixture e. Calculate the decrease in temperature when 6.00 l at 20.0 °c is compressed to 4.00 l. Solve each of the following problems. Web a sample of gas. = (0.76)(2.3 atm) = 1.75 atm p #! What would be the partial pressure of each of the gases in a. A container containing 5.00 l of a gas. Web what is the partial pressure of each of the gases? What are the mole fractions of all the gases in the mixture? Container c (with volume 1.42 dm3) into container d (with volume. Web the partial pressure of a gas can be calculated using the ideal gas law, which we will cover. The partial pressure of each gas 4. Web as the pressure increases,. Web the partial pressure of each gas is 0.265 atm. Web we refer to the pressure. Web calculating partial & total pressures worksheet 1. Web in terms of partial pressures: Relating temperature, volume, and pressure;.
Web A Sample Of Gas.
The volume of the gas particles. Web as the pressure increases,. The total pressure of the mixture e. Calculate the decrease in temperature when 6.00 l at 20.0 °c is compressed to 4.00 l.
Web The Partial Pressure Of Each Gas Is 0.265 Atm.
Web a gas mixture contains oxygen, argon and nitrogen. Web the partial pressure of a gas can be calculated using the ideal gas law, which we will cover. What would be the partial pressure of each of the gases in a. A container containing 5.00 l of a gas.
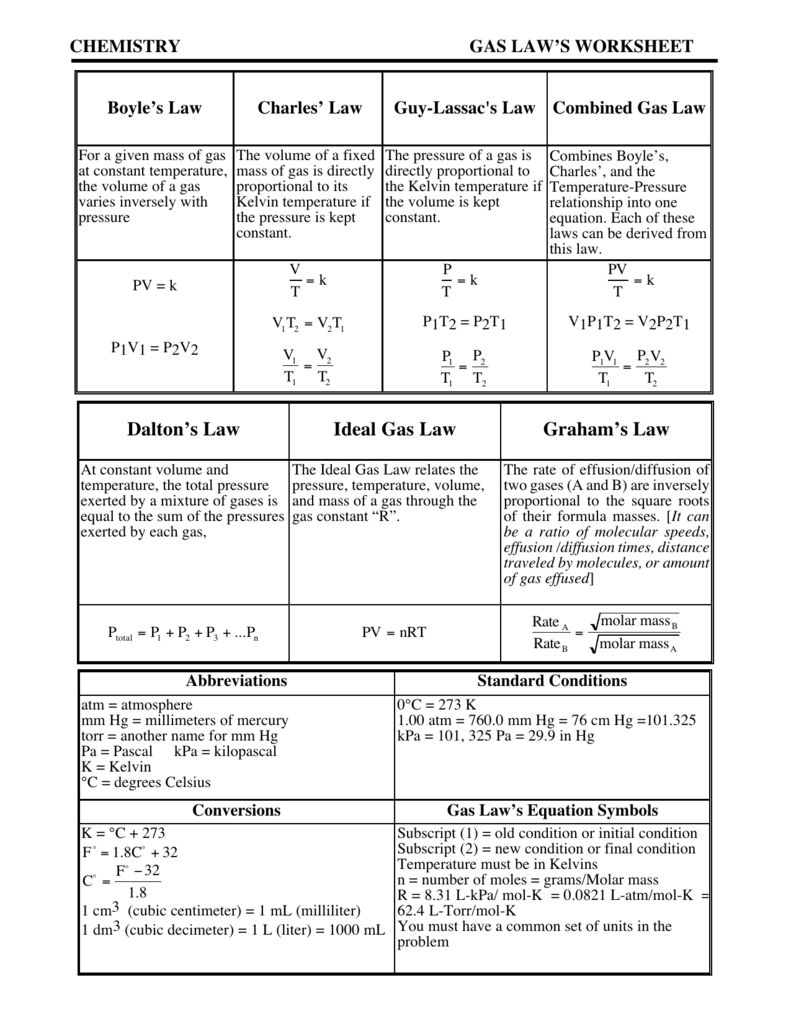
Useful Equations1.00 Atm = 760 Torrpv=Nrtr= 0.0821Dalton’s Law Of.
The mole fraction of each gas d. Web what is the total pressure of the gas? Web the partial pressure of a gas can be calculated using the ideal gas law, which we will cover. The partial pressure of each gas 4.
Keq = Kp = (The Subscript “P” Stands For “Pressure”.) The Numerical Value Of The Two Equilibrium.
Dalton’s law of partial pressure. Solve each of the following problems. Container c (with volume 1.42 dm3) into container d (with volume. Web calculating partial & total pressures worksheet 1.