Molarity Problems Worksheet - To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? The correct option is ‘c’. 0.444 mol of cocl 2 in 0.654 l of solution. M mix = (m 1 v 1 + m 2 v 2) / (v 1 + v 2) = (1.5 x 480) + (1.2 x 520)/ (480 + 520) = (720 + 624)/1000 = 1.344 m q5: Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Use m or mol/l as unit for molarity. Web molarity practice problems #1 1. 2.5 m solution the formula for calculating molarity when the moles of. How many grams of potassium carbonate are needed to make 280 ml of. Web we often want to be able to quantify the amount of a species that is in the solution, which is called the concentration of that.
Molarity Chemistry Tutorial YouTube
Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Web this worksheet and quiz will let you practice the following skills: Web molarity problems worksheet use m or mol/l as unit for molarity. Show all work and circle your final answer. Remember that 1 liter = 1000 ml.
Molarity Problems Worksheet With Answers Worksheetpedia
2) how many liters of 4 m solution can be made using 100 grams of lithium bromide?. Web molarity = _____ problems: M = n (mol)/v (l) first, determines the moles of sodium oxalate: Web this worksheet and quiz will let you practice the following skills: Do not confuse m, l, and ml!
Molarity Practice Worksheet
Remember that 1 liter = 1000 ml. What is the molarity of sodium chloride in sea water? Remember that 1 liter = 1000 ml. To make a 4.00 m solution, how many moles of solute will be. Web we often want to be able to quantify the amount of a species that is in the solution, which is called the.
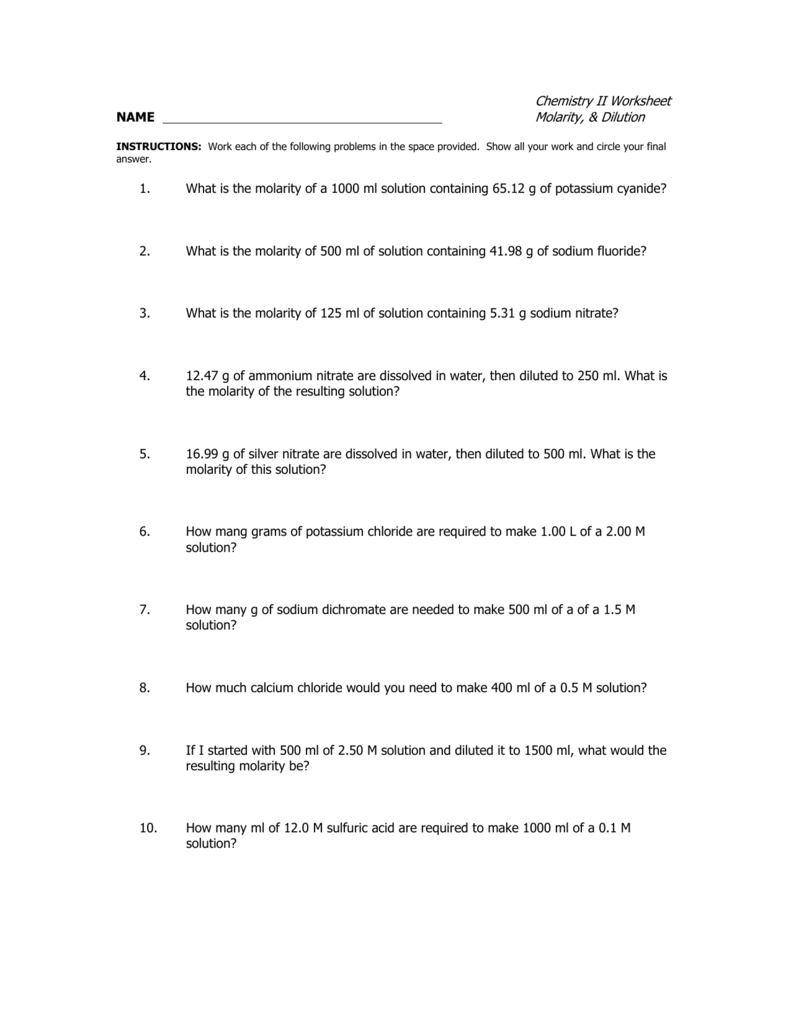
Chemistry II Worksheet NAME Molarity, & Dilution 1. What is the
M = n (mol)/v (l) first, determines the moles of sodium oxalate: Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Web molarity problems worksheet use m or mol/l as unit for molarity. The correct option is ‘c’. Web this worksheet set guides students through the following topics:what is.
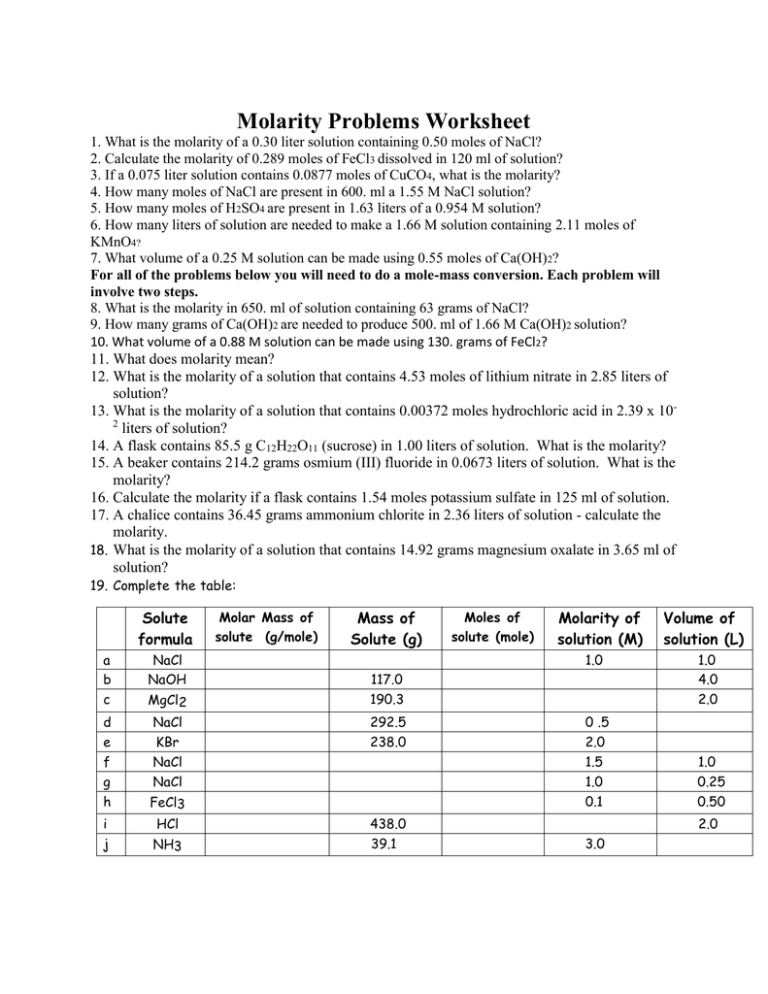
Molarity Problems Worksheet
2) how many liters of 4 m solution can be made using 100 grams of lithium bromide?. Moles of solute 4.00 m = 12.0 l moles of. Web molarity practice problems #1 1. Remember that 1 liter = 1000 ml. To make a 4.00 m solution, how many moles of solute will be.
(PDF) Molarity, Molality and Normailty Emmanuel Gyedu Academia.edu
How many grams of potassium carbonate are needed to make 280 ml of. Web molarity problems worksheet n= # moles. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. M = n (mol)/v (l) first, determines the moles of sodium oxalate: 0.444 mol of cocl 2 in 0.654 l.
Molarity and Stoichiometry Worksheet for 10th Higher Ed Lesson
Web determine the molarity for each of the following solutions: Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Remember that 1 liter = 1000 ml. 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide?. With increase in temperature, which of.
Molarity Practice Problems Worksheet
What is the molarity of a 0.30 liter solution. With increase in temperature, which of these changes? Web this worksheet and quiz will let you practice the following skills: M = n (mol)/v (l) first, determines the moles of sodium oxalate: A) molality b) fraction of solute present in water
Molarity Problems Worksheet
Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. What is the molarity of a 0.30 liter solution. With increase in temperature, which of these changes? Moles of solute 4.00 m = 12.0 l moles of. Do not confuse m, l, and ml!
Molarity Practice Problems Chemistry notes, Chemistry, Chemistry lecture
Web molarity = _____ problems: Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Web molarity practice problems a 2.5 m solution? To make a 4.00 m solution, how many moles of solute will be. V must be in liters (change if necessary) 1.
Show all work and circle your final answer. Moles of solute 4.00 m = 12.0 l moles of. What is the molarity of sodium chloride in sea water? Web molarity practice problems #1 1. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? Web determine the molarity for each of the following solutions: Web this worksheet set guides students through the following topics:what is concentration and molarity? With increase in temperature, which of these changes? Web this worksheet and quiz will let you practice the following skills: How many grams of potassium carbonate are needed to make 280 ml of. Web molarity problems worksheet use m or mol/l as unit for molarity. Web molarity problems worksheet n= # moles. A) molality b) fraction of solute present in water Remember that 1 liter = 1000 ml. Remember that 1 liter = 1000 ml. Web for the first five problems, you need to use the equation that says that the molarity of a solution is equal to the number of moles of. 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide?. Do not confuse m, l, and. Do not confuse m, l, and ml! 0.444 mol of cocl 2 in 0.654 l of solution.
Web We Often Want To Be Able To Quantify The Amount Of A Species That Is In The Solution, Which Is Called The Concentration Of That.
Do not confuse m, l, and. A) molality b) fraction of solute present in water To make a 4.00 m solution, how many moles of solute will be. Web this worksheet set guides students through the following topics:what is concentration and molarity?
Remember That 1 Liter = 1000 Ml.
Web this worksheet and quiz will let you practice the following skills: 2.5 m solution the formula for calculating molarity when the moles of. Web for the first five problems, you need to use the equation that says that the molarity of a solution is equal to the number of moles of. Web molarity practice problems a 2.5 m solution?
Web Molarity Practice Problems #1 1.
Web determine the molarity for each of the following solutions: Web molarity problems worksheet use m or mol/l as unit for molarity. Web molarity = _____ problems: Use m or mol/l as unit for molarity.
Web Solution The Molarity Is Calculated By The Following Formula:
The correct option is ‘c’. The molarity of a mixture, mmix, can be calculated using the following formula: What is the molarity of sodium chloride in sea water? 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide?.