Molarity Practice Problems Worksheet Answers - Select the correct relation between molarity and molality. Determine the molarity of a solution containing 2 mol of ki in 140 ml. How many grams of hydrogen chloride (hcl) are required to prepare 4 litre of 5m hcl in water? A first convert 250 ml to liters, 250/1000 = 0.25 then calculate molarity = 5. Web molarity = ________________ moles of solute. Web molarity practice problems #1 1. To make a 4.00 m solution, how many moles. Web molarity = moles of solute/liters of solution = 8/4 = 2. Web stoichiometry using molarity practice problems answers. Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m.
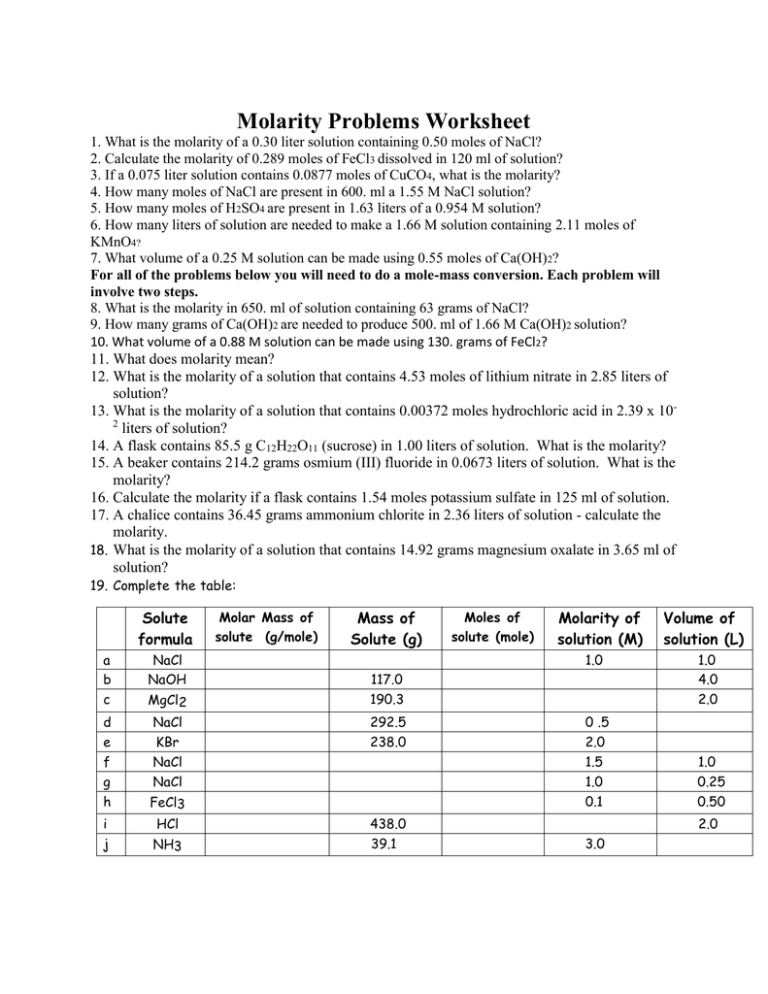
Molarity Problems Worksheet
How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Show all work and circle your final answer. Web solutions to the molarity practice worksheet for the first five problems, you need to use the equation that says that the molarity of. How many grams of hydrogen chloride (hcl) are required to prepare.
43 molarity practice worksheet answer Worksheet Master
A first convert 250 ml to liters, 250/1000 = 0.25 then calculate molarity = 5. How many grams of hydrogen chloride (hcl) are required to prepare 4 litre of 5m hcl in water? Show all work and circle your final answer. Web molarity practice worksheet find the molarity of the following solutions: 1) 0.5 moles of sodium chloride is dissolved.
7 Best Images of Molarity Worksheet With Answers Molality and
Web molarity = moles of solute/liters of solution = 8/4 = 2. Web moles of solute = molarity x volume of solution in litre = (10.0 l) (2.0m) = 20 moles. How many liters of water are needed. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. 2.5 m.
Worksheet Answer Key Molarity Problems Worksheet kidsworksheetfun
You should try to answer the questions without referring to your textbook. Web molarity = moles of solute/liters of solution = 8/4 = 2. Show all work and circle your final answer. Web molarity practice worksheet find the molarity of the following solutions: Select the correct relation between molarity and molality.
worksheet. Molarity Worksheet With Answers. Grass Fedjp Worksheet Study
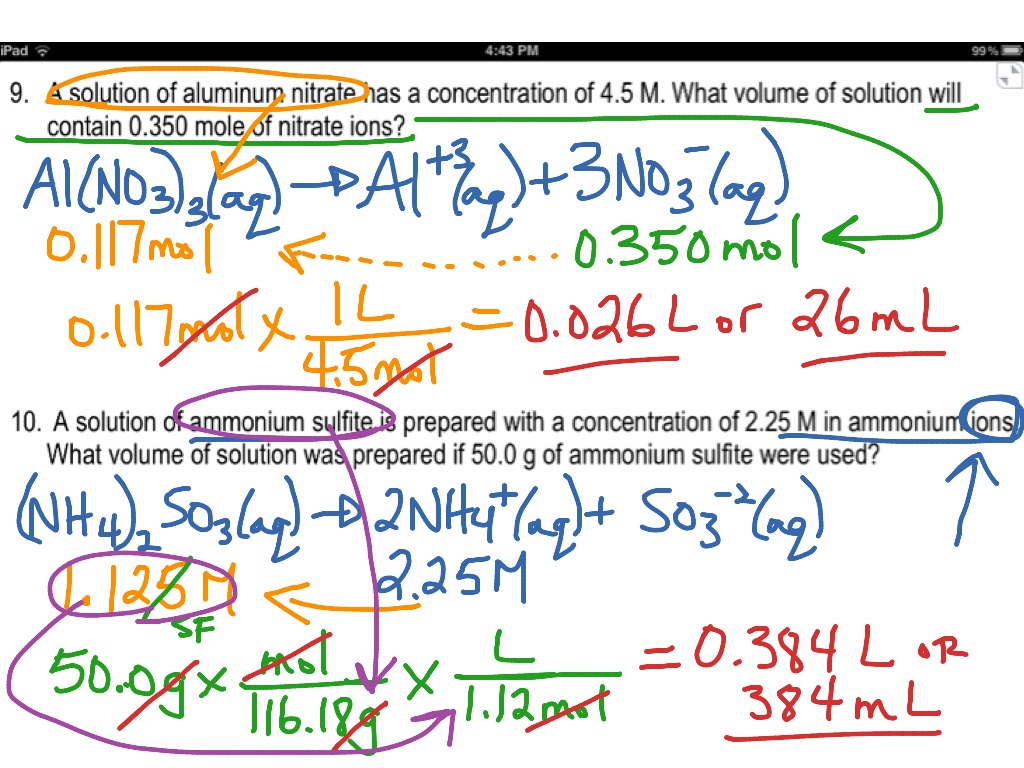
H 2 so 4 + naoh → na 2 so 4 + h 2 o ★if 43 ml of 0 m naoh. Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. You should try to answer the questions without referring to your textbook. To make a 4.00.
Molarity Practice MS MCLARTY'S CLASSES
Determine the molarity of a solution containing 2 mol of ki in 140 ml. 4) 0.5 moles of sodium chloride is dissolved to. Web moles of solute = molarity x volume of solution in litre = (10.0 l) (2.0m) = 20 moles. Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your.
33 Molarity Worksheet Answer Key Chemistry support worksheet
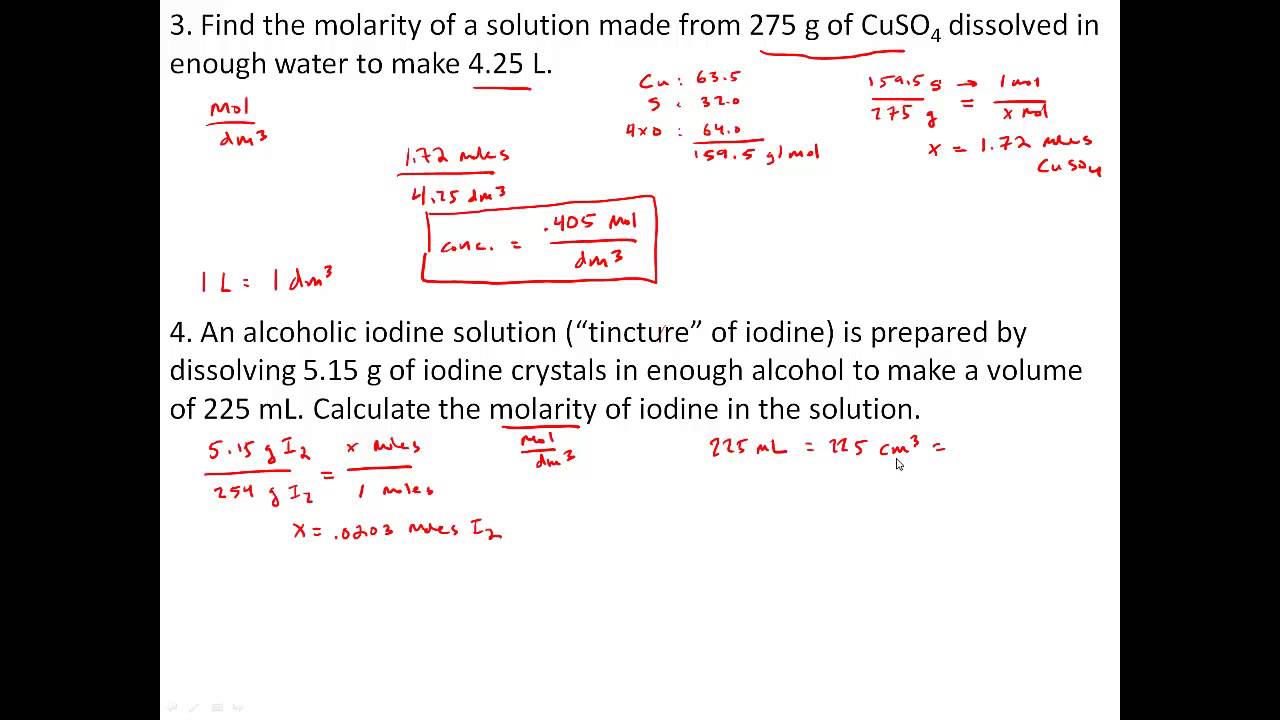
1) 0.5 moles of sodium chloride is dissolved to. To make a 4.00 m solution, how many moles. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Web work in groups on these problems. Select the correct relation between molarity and molality.
Molarity Practice Worksheet Answer Ivuyteq
Web molarity practice problems #1 1. 0.444 mol of cocl 2 in 0.654 l of solution. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Web molarity practice problems created by the steminist teacher a worksheet to help your students practice molarity problems for. You should try to answer.
Molarity Practice Worksheet Answer Ivuyteq
H 2 so 4 + naoh → na 2 so 4 + h 2 o ★if 43 ml of 0 m naoh. Web determine the molarity for each of the following solutions: Thus, there are 20 moles of na 2 co 3 in 10.0l of 2.0m solution. 1) 0.5 moles of sodium chloride is dissolved to. Web molarity = moles.
Molarity Practice Worksheet
[m = molecular weight of solute, d = density g/ml] m ( m 100 + 1. H 2 so 4 + naoh → na 2 so 4 + h 2 o ★if 43 ml of 0 m naoh. Web molarity = ________________ moles of solute. To make a 4.00 m solution, how many moles. Web molarity practice problems #1 1.
Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. Web moles of solute = molarity x volume of solution in litre = (10.0 l) (2.0m) = 20 moles. Web solutions to the molarity practice worksheet for the first five problems, you need to use the equation that says that the molarity of. Web determine the molarity for each of the following solutions: Web molarity = moles of solute/liters of solution = 8/4 = 2. Select the correct relation between molarity and molality. [m = molecular weight of solute, d = density g/ml] m ( m 100 + 1. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. 4) 0.5 moles of sodium chloride is dissolved to. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. H 2 so 4 + naoh → na 2 so 4 + h 2 o ★if 43 ml of 0 m naoh. How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Show all work and circle your final answer. How many grams of hydrogen chloride (hcl) are required to prepare 4 litre of 5m hcl in water? How many liters of water are needed. Thus, there are 20 moles of na 2 co 3 in 10.0l of 2.0m solution. Web molarity practice problems #1 1. Web molarity practice worksheet find the molarity of the following solutions: Web work in groups on these problems. Pipetting and making solutions session 3:
Web Molarity Practice Worksheet Find The Molarity Of The Following Solutions:
How many grams of hydrogen chloride (hcl) are required to prepare 4 litre of 5m hcl in water? Web molarity = ________________ moles of solute. 4) 0.5 moles of sodium chloride is dissolved to. Pipetting and making solutions session 3:
[M = Molecular Weight Of Solute, D = Density G/Ml] M ( M 100 + 1.
Web because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. Web determine the molarity for each of the following solutions: Show all work and circle your final answer. Web work in groups on these problems.
Select The Correct Relation Between Molarity And Molality.
Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7. Web molarity practice problems #1 1. H 2 so 4 + naoh → na 2 so 4 + h 2 o ★if 43 ml of 0 m naoh. Web 1 / 15 flashcards learn test match created by richardsyteacher best used as flashcards as a way to practice molarity.
Web Molarity = Moles Of Solute/Liters Of Solution = 8/4 = 2.
How many liters of water are needed. To make a 4.00 m solution, how many moles. Web solutions to the molarity practice worksheet for the first five problems, you need to use the equation that says that the molarity of. Web molarity practice worksheet find the molarity of the following solutions: