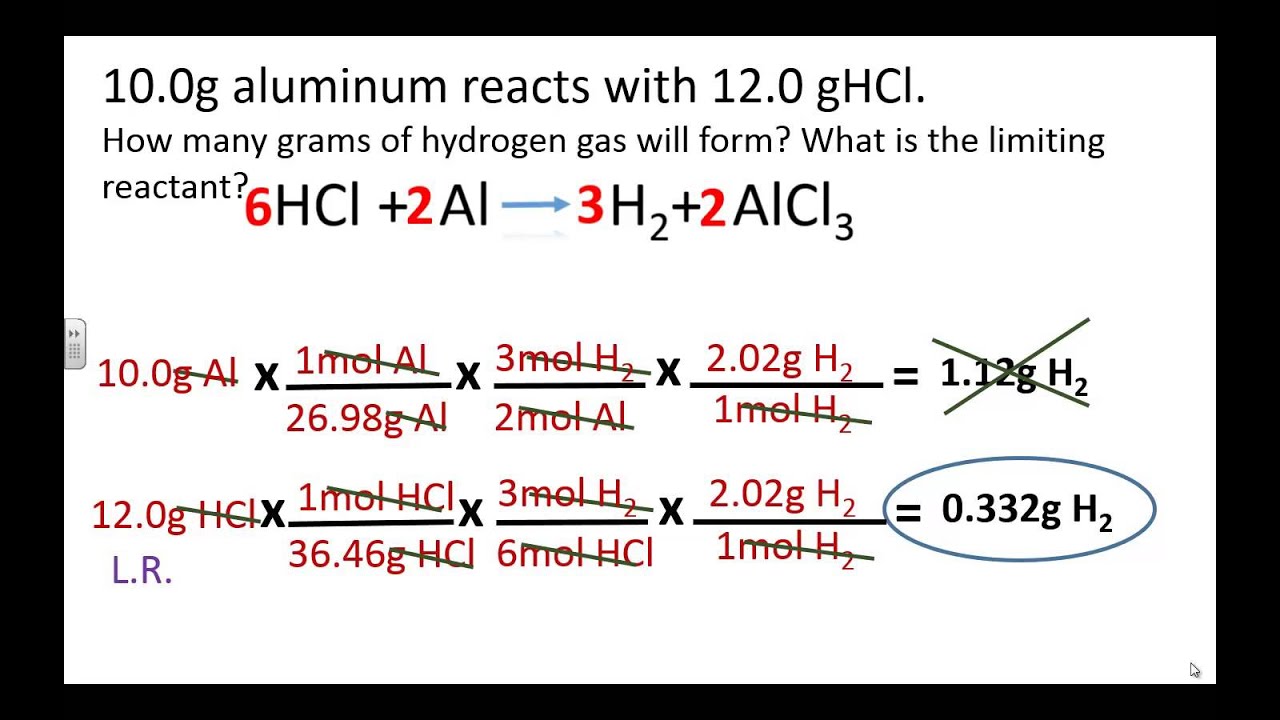
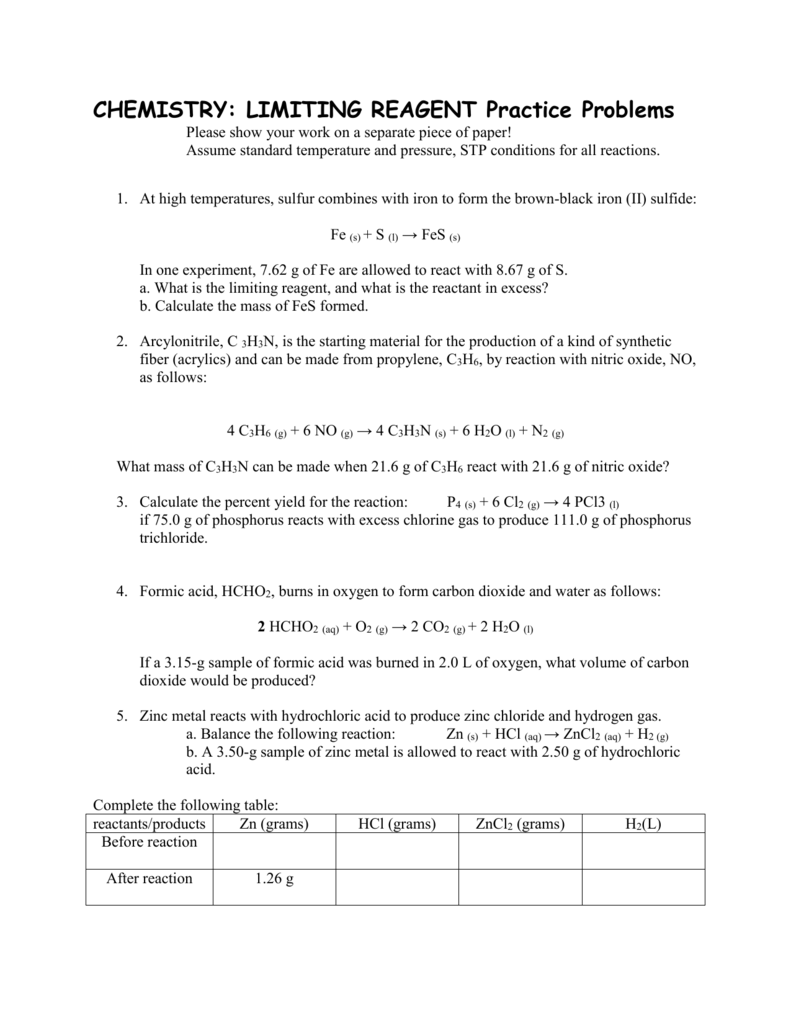
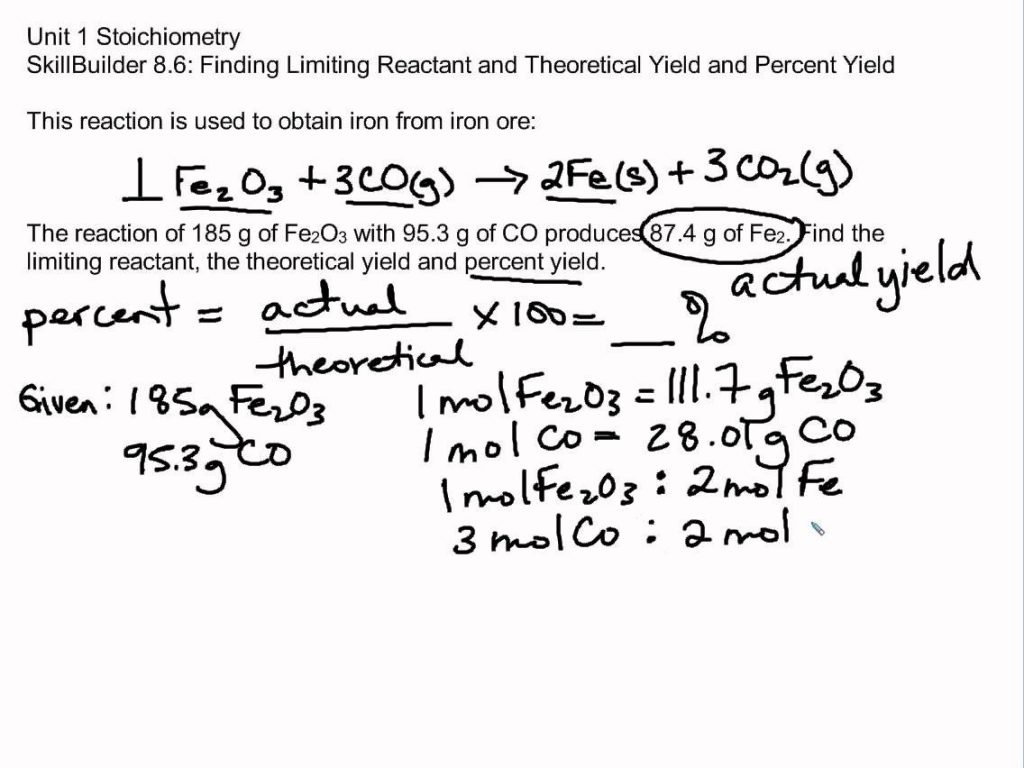
Limiting And Excess Reactants Worksheet Answers - N2 + 3 h2 ( 2 nh3 1. If you have less than you need, this is the limiting reagent (lr). Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation,. Forthe reaction 2s(s) +302(g) ~2s03(g) if6.3 g ofs is. Mass relationships in chemical reactions 4.3 percent yield table of contents. See how much product can be formed by using the maximum amount of the. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Web if you have more than you need, this is the reagent in excess (xs). =0.401!!#$!!# magnesium is limiting and oxygen is in excess 2. Web this worksheet teaches students how to solve a limiting reactant stoichiometry problem, identifying the limiting and the.
Limiting and Excess Reactant Calculations Help YouTube
Our final step is to determine the theoretical yield of \ce {alcl}_3 alcl3 in the reaction. What is a limiting reactant? Limiting & excess reagents 1. Web if you have more than you need, this is the reagent in excess (xs). Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a.
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
=0.401!!#$!!# magnesium is limiting and oxygen is in excess 2. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Web $6.00 zip this ngss aligned lesson begins with a teacher demonstration of a phenomenon that will leave your students. Web if you have more than you need, this is the reagent in excess.
Limiting and Excess Reactants
Web last updated jul 13, 2019 4.1: Web thought question 7.2.1 7.2. See how much product can be formed by using the maximum amount of the. If you have less than you need, this is the limiting reagent (lr). Limiting & excess reagents 1.
Limiting And Excess Reactants Worksheet —
The reactant that is used up first and prevents more product from. Web limiting reagents worksheet 1. If you have less than you need, this is the limiting reagent (lr). Web if you have more than you need, this is the reagent in excess (xs). You have 0.20 mol of.
Limiting Reagent Worksheet Answers Chemical Reactions Sodium
Limiting & excess reagents 1. Web identify the limiting reactant(s) and excess reactant(s). You have 0.20 mol of. Web what is indicated by the maximum amount of precipitate? 3 ml 0.1 m na 2 s neither reagent is limiting and so.
Limiting Reactant Worksheet Stoichiometry 6 Answer Key worksheet
You have 0.20 mol of. If you have less than you need, this is the limiting reagent (lr). =0.401!!#$!!# magnesium is limiting and oxygen is in excess 2. Web identify the limiting reactant(s) and excess reactant(s). Forthe reaction 2s(s) +302(g) ~2s03(g) if6.3 g ofs is.
Limiting Reactant And Percent Yield Worksheet
Forthe reaction 2s(s) +302(g) ~2s03(g) if6.3 g ofs is. How do you know which of two reactants is the. If you have less than you need, this is the limiting reagent (lr). Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation,. Web identify the limiting reactant(s) and excess.
CHEMISTRY LIMITING REAGENT Practice Problems
Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation,. N2 + 3 h2 ( 2 nh3 1. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Web if you have more than you need, this is the reagent in excess (xs)..
Limiting Reactant Worksheet
How do you know which of two reactants is the. Web if you have more than you need, this is the reagent in excess (xs). Our final step is to determine the theoretical yield of \ce {alcl}_3 alcl3 in the reaction. What is a limiting reactant? 3 ml 0.1 m cuso 4 :
Limiting And Excess Reactants Worksheet Thekidsworksheet
If you have less than you need, this is the limiting reagent (lr). 3 ml 0.1 m na 2 s neither reagent is limiting and so. N2 + 3 h2 ( 2 nh3 1. How do you know which of two reactants is the. Web identify the limiting reactant(s) and excess reactant(s).
Web if you have more than you need, this is the reagent in excess (xs). Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation,. Web $6.00 zip this ngss aligned lesson begins with a teacher demonstration of a phenomenon that will leave your students. =0.401!!#$!!# magnesium is limiting and oxygen is in excess 2. 3 ml 0.1 m na 2 s neither reagent is limiting and so. Web if you have more than you need, this is the reagent in excess (xs). Web identify the limiting reactant(s) and excess reactant(s). Web thought question 7.2.1 7.2. Web this worksheet teaches students how to solve a limiting reactant stoichiometry problem, identifying the limiting and the. Web figure out which of the reactants is the limiting reactant or limiting reagent. The reactant that is used up first and prevents more product from. 3 ml 0.1 m cuso 4 : Our final step is to determine the theoretical yield of \ce {alcl}_3 alcl3 in the reaction. Cucl2 + 2 nano3 cu (no3)2 + 2 nacl. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: The limiting reactant is c 2 h 3 br 3 since it would yield the least amount. Say you take a reactant a and calculate the amount of moles of another reactant b required to use up all of a. If you have less than you need, this is the limiting reagent (lr). 1 are the limiting reagents always completely consumed? Forthe reaction 2s(s) +302(g) ~2s03(g) if6.3 g ofs is.
You Have 0.20 Mol Of.
If you have less than you need, this is the limiting reagent (lr). What is a limiting reactant? Web identify the limiting reactant(s) and excess reactant(s). The limiting reactant is c 2 h 3 br 3 since it would yield the least amount.
Cucl2 + 2 Nano3 Cu (No3)2 + 2 Nacl.
See how much product can be formed by using the maximum amount of the. Web $6.00 zip this ngss aligned lesson begins with a teacher demonstration of a phenomenon that will leave your students. Our final step is to determine the theoretical yield of \ce {alcl}_3 alcl3 in the reaction. =0.401!!#$!!# magnesium is limiting and oxygen is in excess 2.
How Do You Know Which Of Two Reactants Is The.
Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation,. Web what is indicated by the maximum amount of precipitate? Mass relationships in chemical reactions 4.3 percent yield table of contents. If you have less than you need, this is the limiting reagent (lr).
Web This Worksheet Teaches Students How To Solve A Limiting Reactant Stoichiometry Problem, Identifying The Limiting And The.
Limiting & excess reagents 1. N2 + 3 h2 ( 2 nh3 1. 3 ml 0.1 m na 2 s neither reagent is limiting and so. 3 ml 0.1 m cuso 4 :