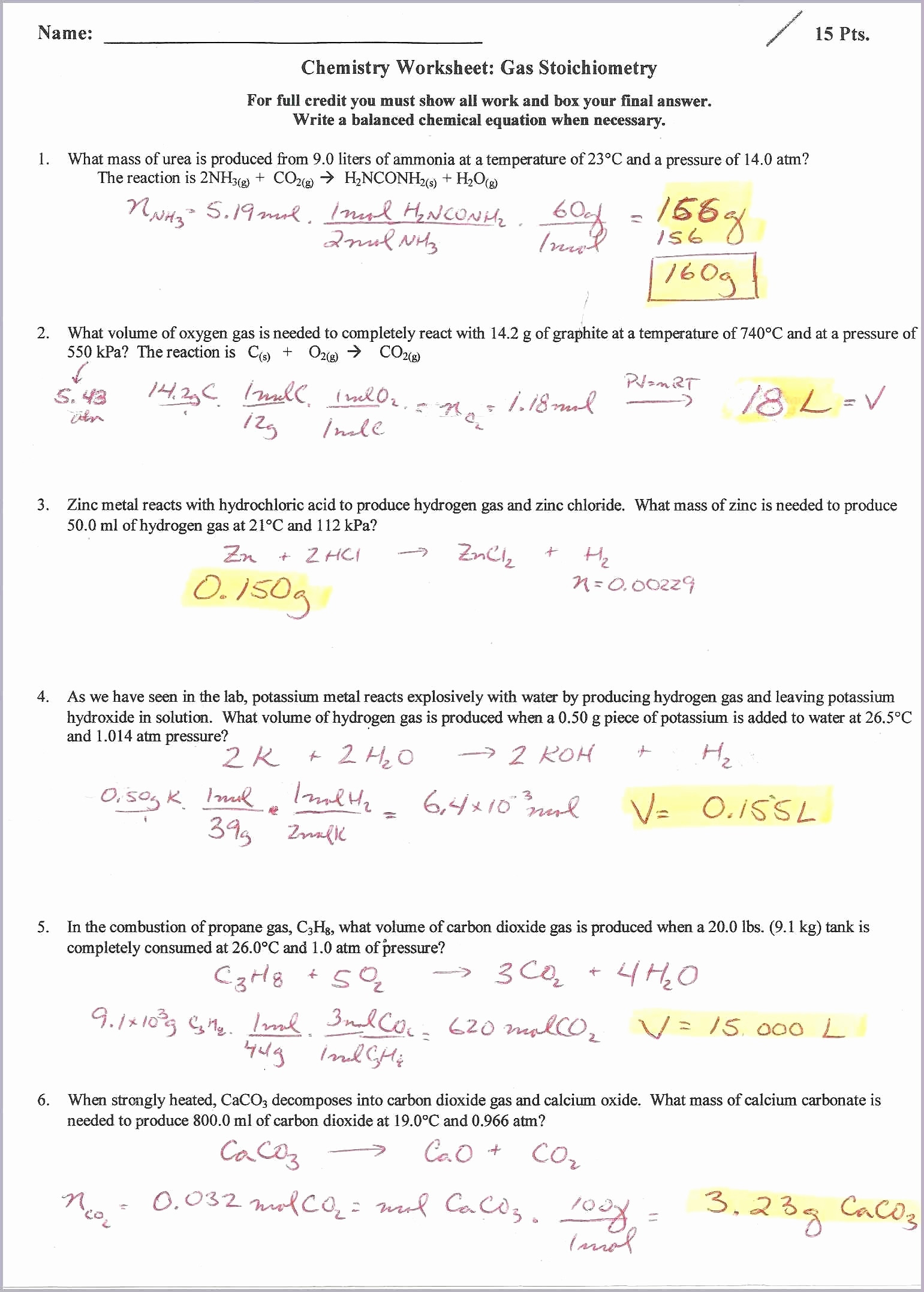
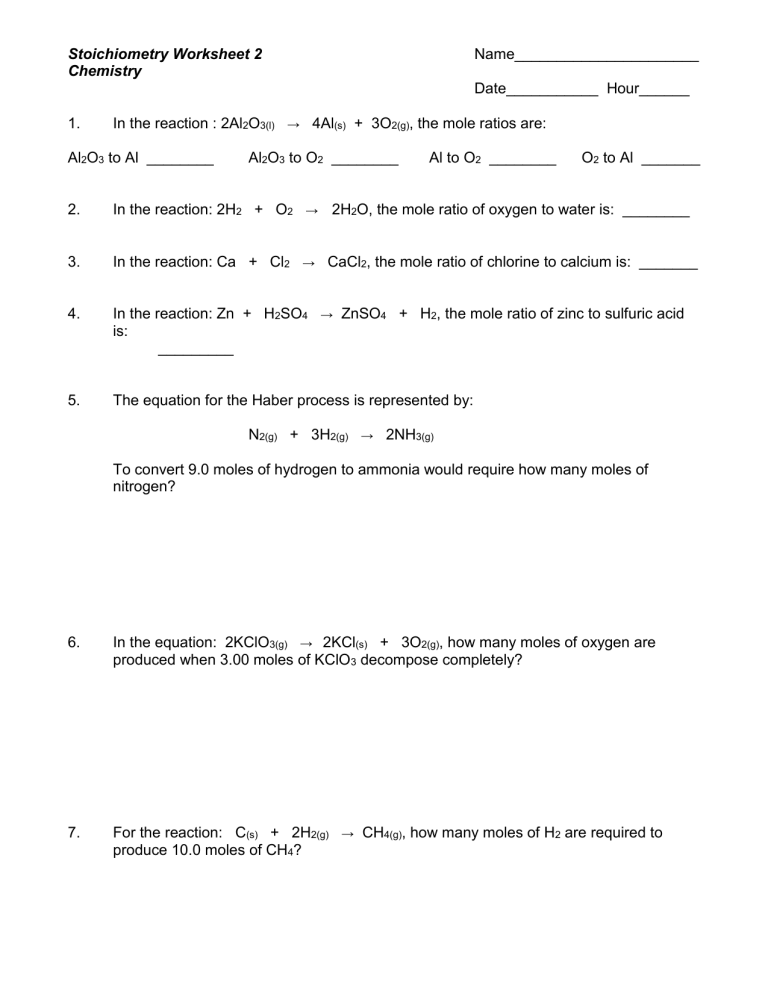
Ideal Gas Law And Stoichiometry Worksheet Answers - Web solutions to the ideal gas law practice worksheet: This worksheet has 10 calculation problems involving the ideal gas law. Web practice ideal gas law calculations that include stoichiometry. How many moles of gas does it. The ideal gas law (pv = nrt) worked example: Web view the full answer. Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and. The ideal gas law relates the four independent physical properties of a gas at any time. This product includes a note sheet to walk your students through the basics of the ideal. Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar.
13 Best Images of Pressure Problems Worksheet Answer Key
Web solutions to the ideal gas law practice worksheet: Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: Ideal gas law practice worksheet solve the following problems using the ideal gas law: Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be. Web.
Solution Stoichiometry Chem Worksheet 15 6
How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of ammonia gas at stp? Ideal gas law practice worksheet solve the following problems using the ideal gas law: Web this product contains 15 pages of multiple choice questions on the ideal gas law, gas stoichiometry, gas reactions, calculating. Web with the ideal gas.
Solved Experiment 13 Name Ideal Gas Law Stoichiometry
1 l 10.0 l nh3 x 2 l. There are no conversions here. Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: The ideal gas law (pv = nrt) worked example: The ideal gas law can be used in stoichiometry problems.
Gas Stoichiometry Worksheet Doc worksheet today
Web worksheet gas stoichiometry worksheet ideal gas law — db excel within ideal gas law worksheet image below, is. Web to solve the following problems: K*mol if pressure is needed in kpa then convert by multiplying by 101.3kpa / 1atm to get =8.31 kpa*l / (k*mole) if i have. Web 1) if 4.00 moles of gasoline are burned, what volume.
Gas Stoichiometry Worksheet Answer Key Thekidsworksheet
Web practice ideal gas law calculations that include stoichiometry. Web to solve the following problems: Using the ideal gas law to calculate number of moles. Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. Web solutions to the ideal gas law practice worksheet:
11 Chemistry Gas Laws Worksheet /
How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa? Web practice ideal gas law calculations that include stoichiometry. Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and. This worksheet has 10 calculation problems involving the ideal.
Gas Law Stoichiometry Worksheet Answers worksheet
Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be. How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2.
Gas Law Stoichiometry not at STP YouTube
Web solutions to the ideal gas law practice worksheet: How many moles of gas does it. Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar. How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa?.
16 Pressure Problems Worksheet Answer Key /
How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa? Using the ideal gas law to calculate number of moles. How many moles of gas does it. Web worksheet gas stoichiometry worksheet ideal gas law — db excel within ideal gas law worksheet image below, is. The ideal gas law.
Stoichiometry Worksheet Answers Worksheets on Gas Stoichiometry
Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar. The ideal gas law can be used in stoichiometry problems. Ideal gas.
Web to solve the following problems: Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be. Web solutions to the ideal gas law practice worksheet: This worksheet has 10 calculation problems involving the ideal gas law. K*mol if pressure is needed in kpa then convert by multiplying by 101.3kpa / 1atm to get =8.31 kpa*l / (k*mole) if i have. Web view the full answer. Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to. 1 l 10.0 l nh3 x 2 l. Using the ideal gas law to calculate number of moles. Web practice ideal gas law calculations that include stoichiometry. The ideal gas law states that pv=nrt, where p is the pressure of a gas,. Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and. This product includes a note sheet to walk your students through the basics of the ideal. Ideal gas law practice worksheet solve the following problems using the ideal gas law: The ideal gas law relates the four independent physical properties of a gas at any time. How many moles of gas does it. The ideal gas law can be used in stoichiometry problems. The ideal gas law (pv = nrt) worked example: How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa?
Web Practice Ideal Gas Law Calculations That Include Stoichiometry.
1 l 10.0 l nh3 x 2 l. The ideal gas law states that pv=nrt, where p is the pressure of a gas,. The ideal gas law can be used in stoichiometry problems. This worksheet has 10 calculation problems involving the ideal gas law.
Web View The Full Answer.
How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa? Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be. Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: This product includes a note sheet to walk your students through the basics of the ideal.
Web Worksheet Gas Stoichiometry Worksheet Ideal Gas Law — Db Excel Within Ideal Gas Law Worksheet Image Below, Is.
How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of ammonia gas at stp? Web to solve the following problems: Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar. There are no conversions here.
How Many Moles Of Gas Does It.
Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and. Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition. Using the ideal gas law to calculate number of moles. K*mol if pressure is needed in kpa then convert by multiplying by 101.3kpa / 1atm to get =8.31 kpa*l / (k*mole) if i have.