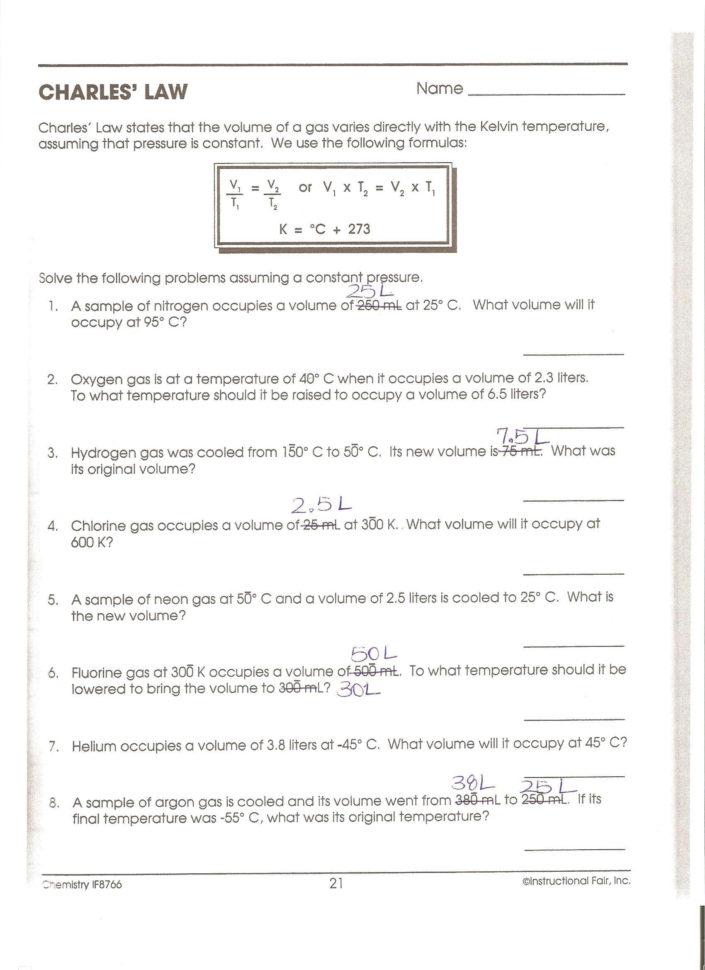
Gas Law Worksheet 2 - The product of a reaction, h2 gas, was collected in a bottle. What is the pressure of the gas in torr if the gas is at 25oc? Web mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. Ideal gas law worksheet pv = nrt. 1 atm = 760.0 mm hg = 101.3 kpa. A gas has a volume of 39 liters at stp. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web gas law worksheet 2. Apply gas laws to calculations. Web gas laws 2 1.
gas laws worksheet 1
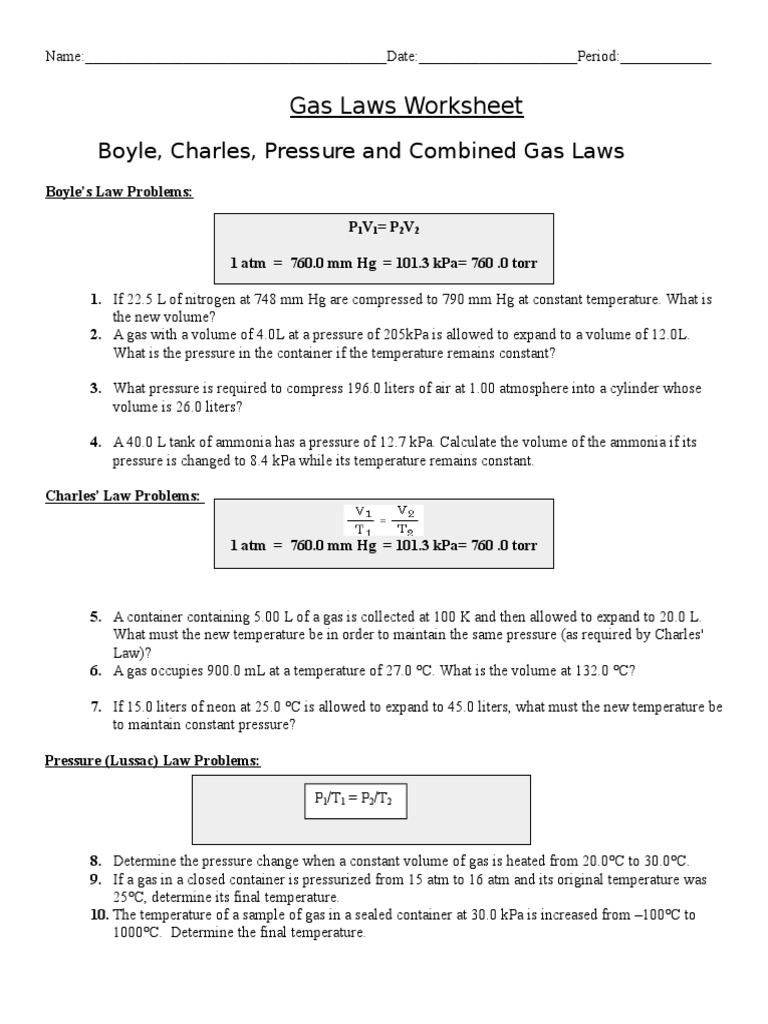
Web 1 t 2= v 2 t 1 1 1 = boyle’s lawcombined gas law pv = k p1v1 = p2v2 the pressure of a gas is directly proportional to the kelvin temperature if the volume. If the gases are at stp, what are the partial. Students will solve for each of the variables, and for. Web a good worksheet.
Gas Laws Worksheet
If the gases are at stp, what are the partial. Web this was avogadro’s great contribution to chemistry, as lothar meyer so dramatically declared in the quote above. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law! Web 1 t 2= v 2 t 1 1.
Gas Law Practice Worksheet kamberlawgroup
Web a gas mixture contains three components: But if the two gases are chemically different, the masses of the samples will be different. Web gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems: The product of a reaction, h2 gas, was collected in a bottle. The simulation (15 minutes) we are going to.
Foothill High School
Web this was avogadro’s great contribution to chemistry, as lothar meyer so dramatically declared in the quote above. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law! Determine the total pressure of a gas mixture that. A gas has a volume of 39 liters at stp..
Gas Laws Worksheet 2 Boyles Charles and Combined PDF Gases Pressure
1 atm = 760.0 mm hg = 101.3 kpa. But if the two gases are chemically different, the masses of the samples will be different. P t = p 1 + p 2 + p 3 +. 28 g \(co\), 2.0 g \(h_2\) and 8.0 g of \(o_2\). Determine the total pressure of a gas mixture that.
Gas Laws Worksheet 2 Chimp Wiring
Web gas laws packet #2. If 22.5 l of nitrogen at 748 mm hg are. What will its volume be at 4 atm and 25 oc? Complete all three problems and identify the correct gas law. Web gas laws 2 1.
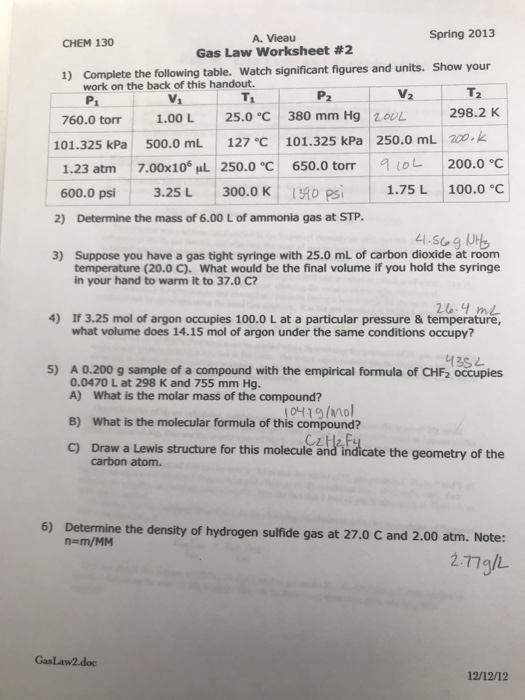
Solved Spring 2013 A. Vieau Gas Law worksheet 2 CHEM 130
Ideal gas law worksheet pv = nrt. If 22.5 l of nitrogen at 748 mm hg are. But if the two gases are chemically different, the masses of the samples will be different. 1) how many moles of gas does it take to. What will its volume be at 4 atm and 25 oc?
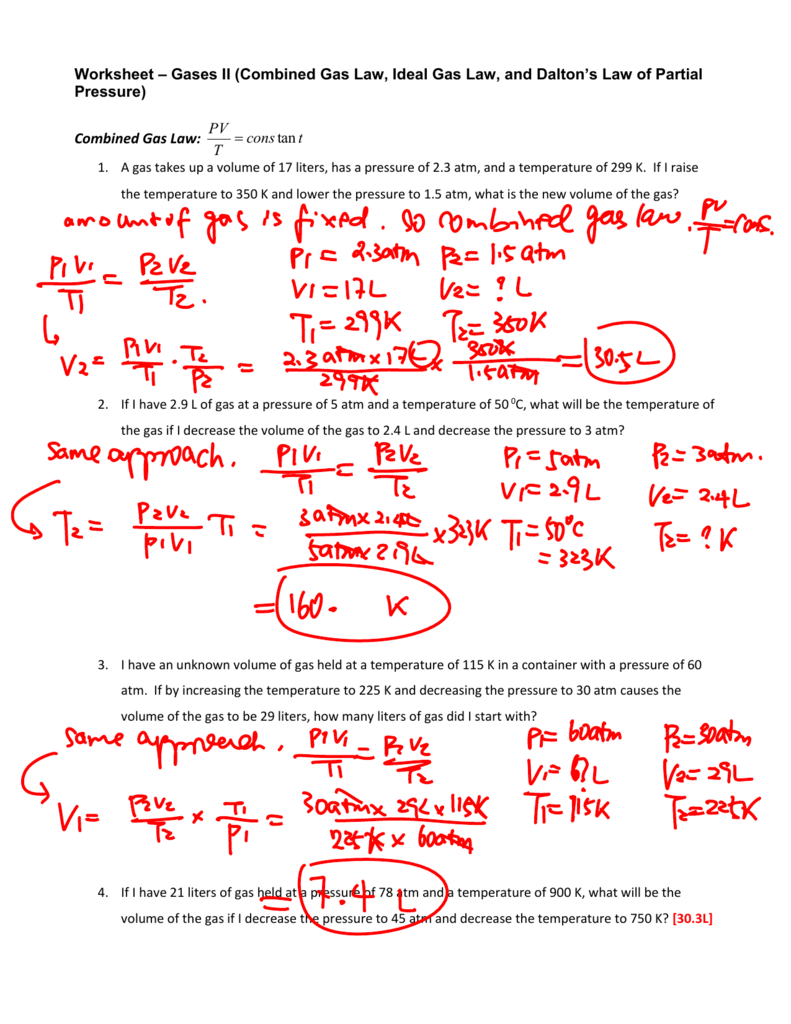
Worksheet Gas Laws II Answers
A gas has a volume of 39 liters at stp. Web gas laws 2 (worksheet) solutions. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web r = 0.08206 l × atm/k × mol k = o c + 273.15 gas densities from pv = nrt we can see that if two gas samples have the.
Ideal Gas Law Worksheet
Apply gas laws to calculations. Web r = 0.08206 l × atm/k × mol k = o c + 273.15 gas densities from pv = nrt we can see that if two gas samples have the same pressure, volume, and temperature, they must have the same number of moles. P t = p 1 + p 2 + p 3.
Worksheet On Ideal Gas Equation Ideal Gas Laws Worksheet worksheet
A gas has a volume of 39 liters at stp. Web mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. What is the pressure of the gas in torr if the gas is at 25oc? Web gas laws worksheet atm = 760.0 mm hg =.
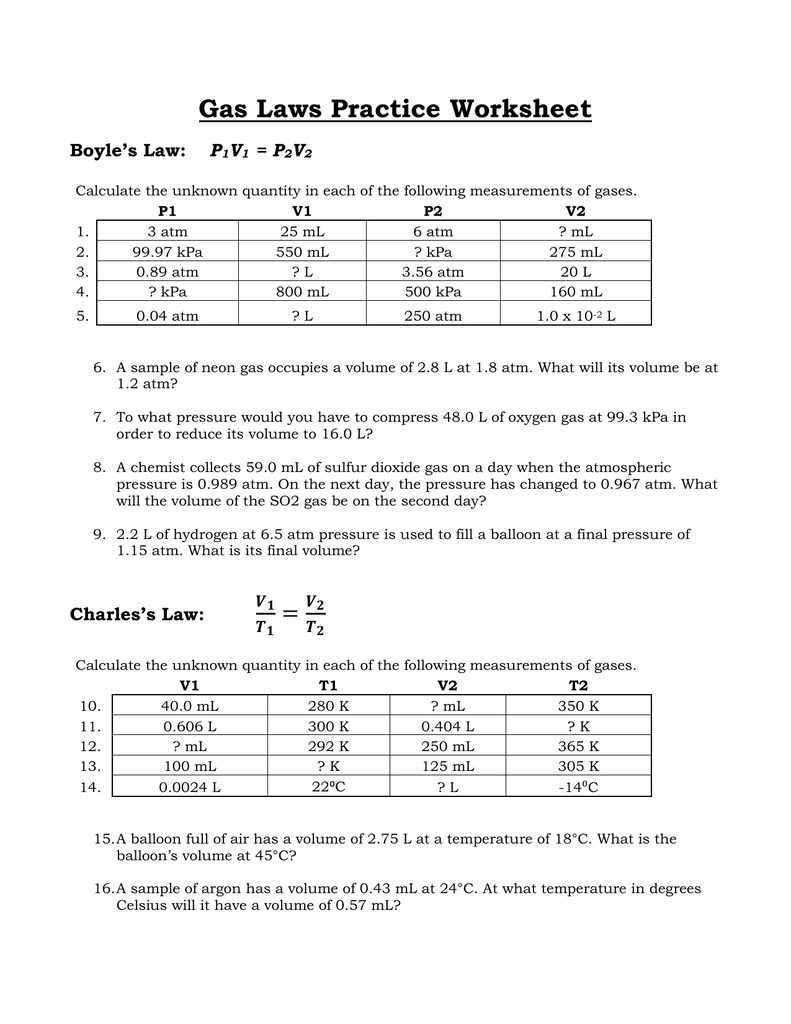
1) how many moles of gas does it take to. Web gas laws worksheet 2. Ml of a gas is contained. Web mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. Web gas laws packet #2. 1 atm = 760.0 mm hg = 101.3 kpa. A gas has a volume of 39 liters at stp. The simulation (15 minutes) we are going to study 2 of the famous gas laws: Students will solve for each of the variables, and for. Web r = 0.08206 l × atm/k × mol k = o c + 273.15 gas densities from pv = nrt we can see that if two gas samples have the same pressure, volume, and temperature, they must have the same number of moles. Web gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems: Web these popular skills practice worksheets help your students master combined gas law. P t = p 1 + p 2 + p 3 +. Web 1 t 2= v 2 t 1 1 1 = boyle’s lawcombined gas law pv = k p1v1 = p2v2 the pressure of a gas is directly proportional to the kelvin temperature if the volume. Web this was avogadro’s great contribution to chemistry, as lothar meyer so dramatically declared in the quote above. 28 g \(co\), 2.0 g \(h_2\) and 8.0 g of \(o_2\). Ideal gas law worksheet pv = nrt. Complete all three problems and identify the correct gas law. Simulation worksheet 2 screen 3: What will its volume be at 4 atm and 25 oc?
Web Ideal Gas Law Practice Worksheet Solve The Following Problems Using The Ideal Gas Law:
Web gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems: If the gases are at stp, what are the partial. A gas has a volume of 39 liters at stp. Apply gas laws to calculations.
Determine The Total Pressure Of A Gas Mixture That.
Ml of a gas is contained. Simulation worksheet 2 screen 3: Web this was avogadro’s great contribution to chemistry, as lothar meyer so dramatically declared in the quote above. What is the pressure of the gas in torr if the gas is at 25oc?
Complete All Three Problems And Identify The Correct Gas Law.
Web these popular skills practice worksheets help your students master combined gas law. Web gas laws worksheet 2. What will its volume be at 4 atm and 25 oc? Web 1 t 2= v 2 t 1 1 1 = boyle’s lawcombined gas law pv = k p1v1 = p2v2 the pressure of a gas is directly proportional to the kelvin temperature if the volume.
If 22.5 L Of Nitrogen At 748 Mm Hg Are.
If 22.5 l of nitrogen at 748. Web a gas mixture contains three components: Ideal gas law worksheet pv = nrt. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law!