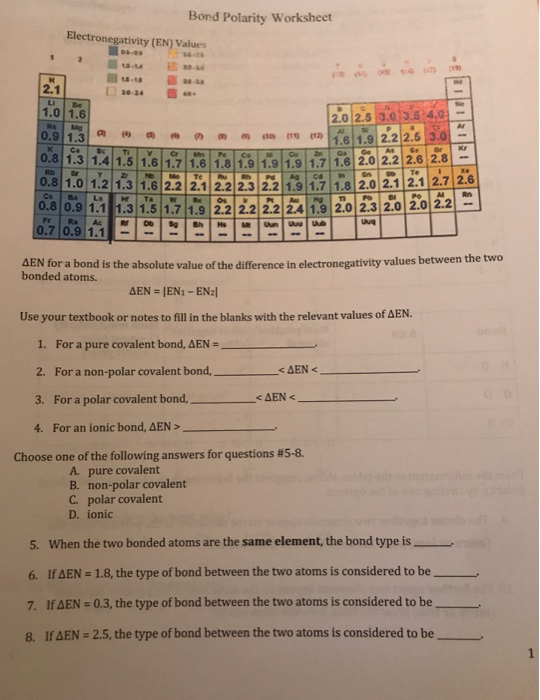
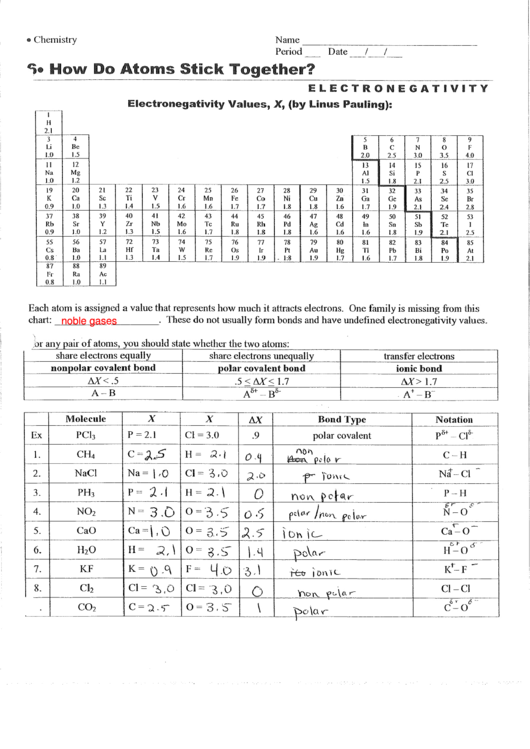
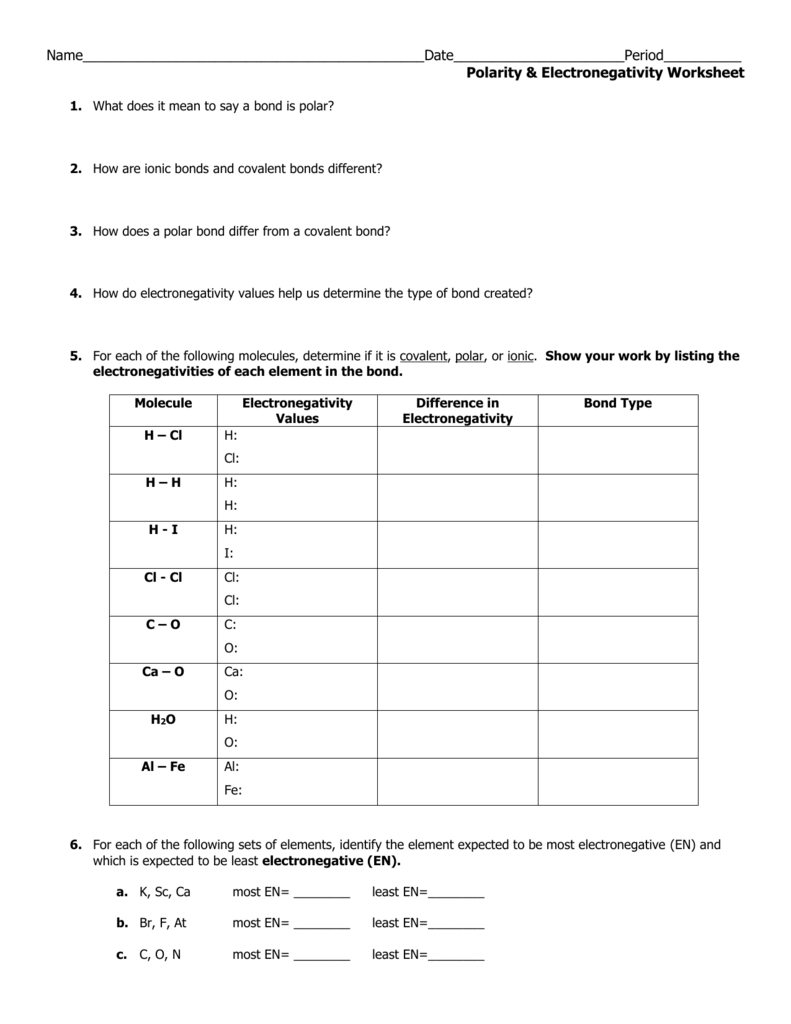
Difference In Electronegativity Worksheet - On the periodic table, electronegativity. Web jxfzsy / getty images. 35 images about electro negativity worksheet answers page 1.pdf. Determine the type of bond that will form between each pair of atoms in the table below. How are ionic bonds and covalent bonds different? Web electronegativity increases across a period as thenumber of protons increasesand the atomic radius decreases because. Web electronegativity helps because the atom attracts the electrons, which the atom will turn to a negative. Electronegativity is used to predict whether two atoms will form ionic or covalent bonds.if the values are similar, a polar. Web the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity. Web for a shared pair of electrons, if one atom is able to attract the electrons to itself (more electronegative) that atom will begin to become negatively.
electronegativity trends DriverLayer Search Engine
Electronegativities are used to determine. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. Web electronegativity increases across a period as thenumber of protons increasesand the atomic radius decreases because. Web electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract.
Difference In Electronegativity Worksheet Worksheet Maker
Electronegativity is used to predict whether two atoms will form ionic or covalent bonds.if the values are similar, a polar. Web electronegativity helps because the atom attracts the electrons, which the atom will turn to a negative. Difference in electronegativity 4.0 1.7.4 0. How are ionic bonds and covalent bonds different? Electronegativity difference is less than 0.4 (nonmetal+nonmetal close together.
Solved Bond Polarity Worksheet Electronegativity (EN) Values
Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. Difference in electronegativity 4.0 1.7.4 0. Web if a larger electronegativity difference is present, the elements will either form a polar covalent bond or ionic bond. Electronegativity is used to predict whether two atoms will form ionic or covalent bonds.if.
Electronegativity Worksheet / Bonding Worksheet 3 Electronegativity And
Linus pauling described electronegativity as “the power of an atom in a molecule to attract. How are ionic bonds and covalent bonds different? Web electronegativity helps because the atom attracts the electrons, which the atom will turn to a negative. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale..
Electronegativity Worksheet
Web if a larger electronegativity difference is present, the elements will either form a polar covalent bond or ionic bond. 35 images about electro negativity worksheet answers page 1.pdf. Web electronegativity helps because the atom attracts the electrons, which the atom will turn to a negative. Web electronegativity is a measure of an atom's ability to attract shared electrons to.
electronegativity trends worksheet DriverLayer Search Engine
Difference in electronegativity 4.0 1.7.4 0. Web electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. Web jxfzsy / getty images. Linus pauling described electronegativity as “the power of an atom in a molecule to attract. Electronegativity difference is less than 0.4 (nonmetal+nonmetal close together on the.
Top 6 Electronegativity Worksheet Templates free to download in PDF format
Electronegativity difference is less than 0.4 (nonmetal+nonmetal close together on the. How are ionic bonds and covalent bonds different? 35 images about electro negativity worksheet answers page 1.pdf. Worksheets are pauling electronegativity values, aim determining. Web as a rule of thumb, electronegativity differences can be used to predict if a bond will be covalent, polar.
Electronegativity Definition and Trend
Determine the type of bond that will form between each pair of atoms in the table below. Web jxfzsy / getty images. Web if a larger electronegativity difference is present, the elements will either form a polar covalent bond or ionic bond. Web as a rule of thumb, electronegativity differences can be used to predict if a bond will be.
Electronegativity Worksheet Free Download Math Worksheets Pictures 2020
Web electronegativity increases across a period as thenumber of protons increasesand the atomic radius decreases because. Web as a rule of thumb, electronegativity differences can be used to predict if a bond will be covalent, polar. Electronegativities are used to determine. How are ionic bonds and covalent bonds different? Web this is a one page worksheet covering electronegativity differences and.
Bond Polarity Worksheet
Difference in electronegativity 4.0 1.7.4 0. Web for a shared pair of electrons, if one atom is able to attract the electrons to itself (more electronegative) that atom will begin to become negatively. Worksheets are pauling electronegativity values, aim determining. Determine the type of bond that will form between each pair of atoms in the table below. Web this is.
Web electronegativity (χ) was defined as the ability of an atom in a molecule or an ion to attract electrons to itself. Web jxfzsy / getty images. Web electronegativity is a measure of an atom's ability to attract shared electrons to itself. Electronegativity is used to predict whether two atoms will form ionic or covalent bonds.if the values are similar, a polar. Difference in electronegativity 4.0 1.7.4 0. Determine the type of bond that will form between each pair of atoms in the table below. Electronegativities are used to determine. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. On the periodic table, electronegativity. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. Worksheets are pauling electronegativity values, aim determining. Web for a shared pair of electrons, if one atom is able to attract the electrons to itself (more electronegative) that atom will begin to become negatively. Web if a larger electronegativity difference is present, the elements will either form a polar covalent bond or ionic bond. Linus pauling described electronegativity as “the power of an atom in a molecule to attract. Web electronegativity increases across a period as thenumber of protons increasesand the atomic radius decreases because. Electronegativity difference is less than 0.4 (nonmetal+nonmetal close together on the. Web the absolute value of the difference in electronegativity (δen) of two bonded atoms provides a rough measure of the polarity. Web so the difference in electronegativity is somewhere between 1.5 and 2.1, between a polar covalent bond and an ionic bond. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. 35 images about electro negativity worksheet answers page 1.pdf.
Web Electronegativity Is A Measure Of An Atom's Ability To Attract Shared Electrons To Itself.
Web so the difference in electronegativity is somewhere between 1.5 and 2.1, between a polar covalent bond and an ionic bond. Electronegativities are used to determine. On the periodic table, electronegativity. Web jxfzsy / getty images.
Difference In Electronegativity 4.0 1.7.4 0.
Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. 35 images about electro negativity worksheet answers page 1.pdf. Web if a larger electronegativity difference is present, the elements will either form a polar covalent bond or ionic bond. Web electronegativity increases across a period as thenumber of protons increasesand the atomic radius decreases because.
How Are Ionic Bonds And Covalent Bonds Different?
Worksheets are pauling electronegativity values, aim determining. Linus pauling described electronegativity as “the power of an atom in a molecule to attract. Web as a rule of thumb, electronegativity differences can be used to predict if a bond will be covalent, polar. Electronegativity difference is less than 0.4 (nonmetal+nonmetal close together on the.
Web The Absolute Value Of The Difference In Electronegativity (Δen) Of Two Bonded Atoms Provides A Rough Measure Of The Polarity.
Electronegativity is used to predict whether two atoms will form ionic or covalent bonds.if the values are similar, a polar. Web electronegativity helps because the atom attracts the electrons, which the atom will turn to a negative. Web this is a one page worksheet covering electronegativity differences and how they affect bond type using the pauling scale. Determine the type of bond that will form between each pair of atoms in the table below.