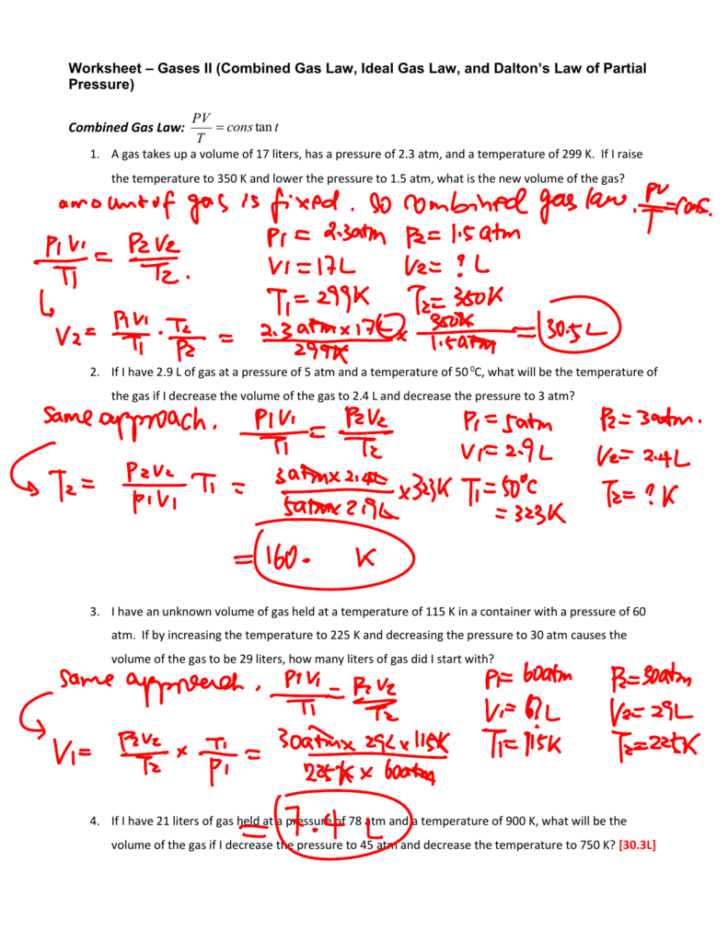
Combined Gas Law Worksheet - The pressure of a gas. The combined gas law solve the following problems. Web combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? Determine the total pressure of a gas mixture that contains oxygen at a. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web combined gas law problems: 2) a toy balloon has. P t = p 1 + p 2 + p 3 +. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. As always, include enough work and show the units to ensure full credit.
Combined Gas Law Problems Worksheet
Determine the total pressure of a gas mixture that contains oxygen at a. 2) a toy balloon has. As always, include enough work and show the units to ensure full credit. Web combined gas law problems: 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc.
Combined Gas Law Problems Worksheet Answers —
P t = p 1 + p 2 + p 3 +. As always, include enough work and show the units to ensure full credit. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. The combined gas.
Gas Laws worksheet
A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. Determine the total pressure of a gas mixture that contains oxygen at a. P t = p 1 + p 2 + p 3 +. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web combined gas.
worksheet. Combined Gas Law Worksheet Answers. Worksheet Fun Worksheet
Determine the total pressure of a gas mixture that contains oxygen at a. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. The combined gas law solve the following problems. Web combined gas law problems: The pressure of a gas.
Combined Gas Law Problems Worksheet —
As always, include enough work and show the units to ensure full credit. Web combined gas law problems: 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. Determine the total pressure of a gas mixture that contains oxygen at a. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law:
Gas Law Worksheet 2
Web combined gas law problems: As always, include enough work and show the units to ensure full credit. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. The pressure of a gas. P t = p 1 + p 2 + p 3 +.
Combined Gas Law Problems Worksheet
As always, include enough work and show the units to ensure full credit. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. 2) a toy balloon has. Web combined gas law problems:
Combined Gas Law Worksheet Answer Key worksheet
P t = p 1 + p 2 + p 3 +. The combined gas law solve the following problems. Web combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? 2) a toy balloon has. The.
Combined Gas Law Worksheet
Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: The combined gas law solve the following problems. P t = p 1 + p 2 + p 3 +. As always, include enough work and show the units to ensure full credit. A gas balloon has a volume of 106.0 liters when the temperature is 45.0.
Lines Combined Gas Law Worksheet Chart Answer Key Chart —
Determine the total pressure of a gas mixture that contains oxygen at a. P t = p 1 + p 2 + p 3 +. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. Web combined gas law worksheet 1) if i initially have 4.0 l of a gas at.
Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: Web combined gas law problems: Determine the total pressure of a gas mixture that contains oxygen at a. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. 2) a toy balloon has. Web combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? The combined gas law solve the following problems. The pressure of a gas. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. P t = p 1 + p 2 + p 3 +. As always, include enough work and show the units to ensure full credit.
Web Combined Gas Law Problems:
P t = p 1 + p 2 + p 3 +. 2) a toy balloon has. Web combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is.
Determine The Total Pressure Of A Gas Mixture That Contains Oxygen At A.
The pressure of a gas. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. As always, include enough work and show the units to ensure full credit. Web gas law worksheet #2 (dalton’s law and ideal gas law) dalton’s law: