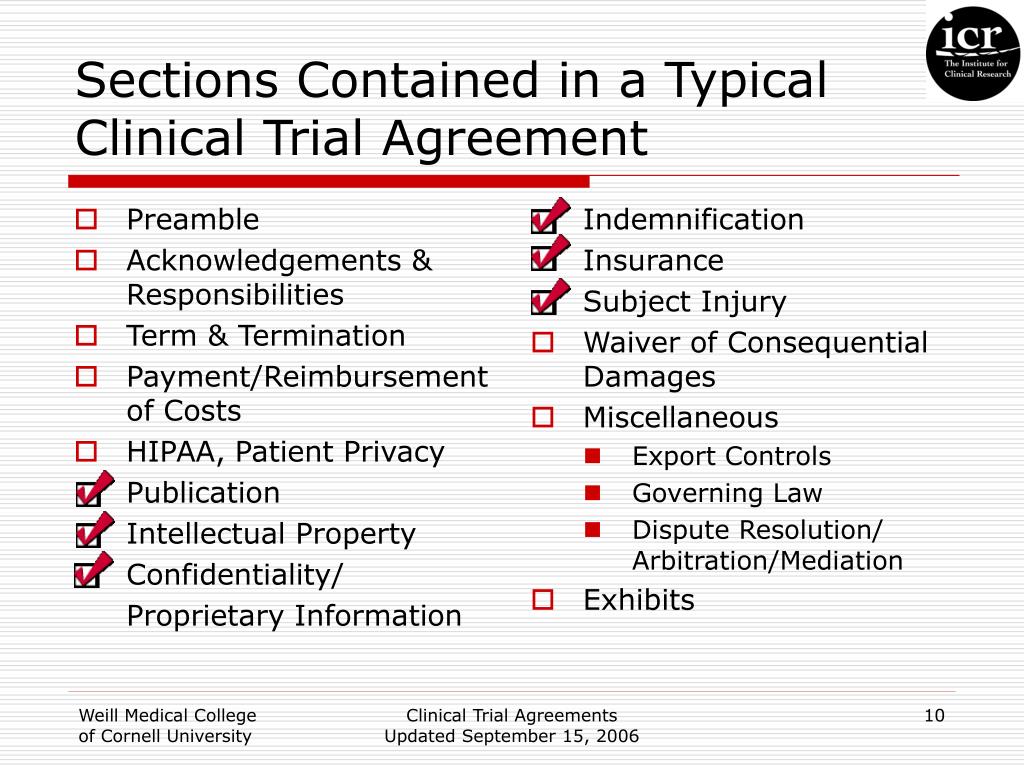
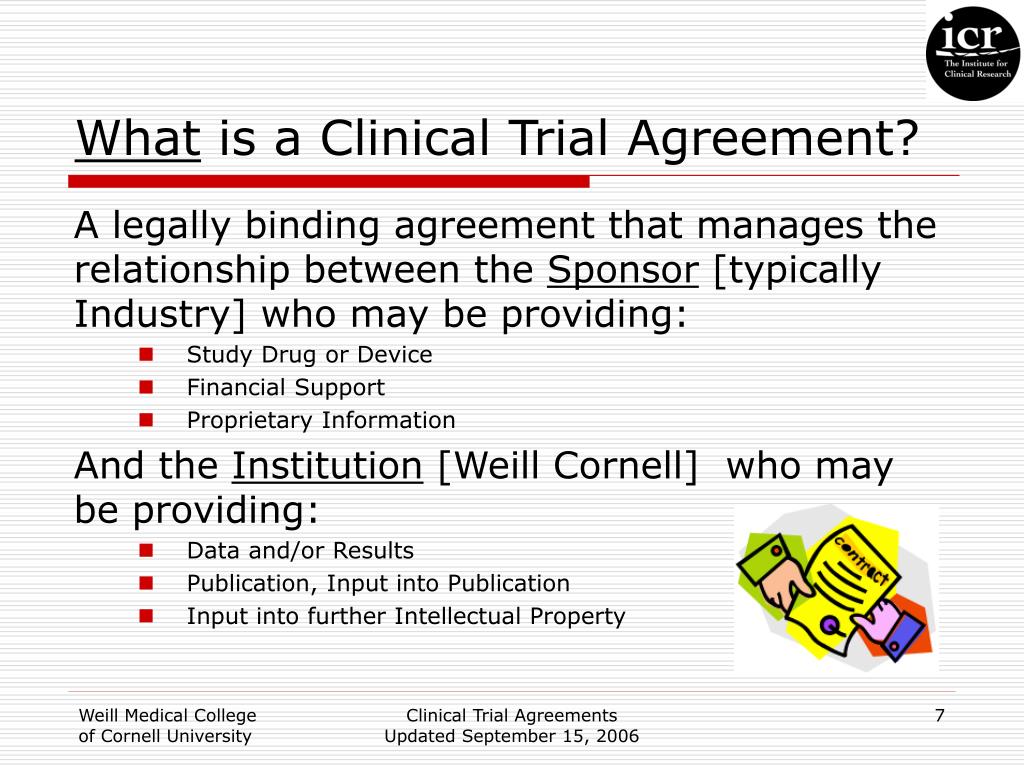
Clinical Trial Agreement Template - Changes in the protocol ay be m ade only. Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Site shall, and shall cause principal investigator to, diligently. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the.
Free Clinical Trial Agreement Free to Print, Save & Download
Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. Site shall, and shall cause principal investigator to, diligently. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the accelerated clinical trial agreement (acta) was prepared with the.
PPT Clinical Trial Agreements PowerPoint Presentation, free download
In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. Web.
TEMPLATE CLINICAL TRIAL AGREEMENT
Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Changes in the protocol ay be m ade only. Web a clinical.
FREE 44+ Sample Checklist Samples & Templates in Samples in Excel PDF
Site shall, and shall cause principal investigator to, diligently. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the..
OHSU Clinical Trial Agreement Template
Site shall, and shall cause principal investigator to, diligently. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Changes in the protocol ay be m ade only. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the three major.
Clinical Trial Agreement Contract Template (with Sample)
Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Site shall, and shall cause principal investigator to, diligently. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry.
Template Clinical Trial Agreement Ccmo HQ Printable Documents
Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. Site shall, and shall cause principal investigator to, diligently..
Clinical Trial Agreement, Sample Clinical Trial Agreement Template
Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Changes.
Clinical study agreement template in Word and Pdf formats page 2 of 7
Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. Changes in the protocol ay be m ade only. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Web a clinical.
PPT Clinical Trial Agreements PowerPoint Presentation, free download
Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Changes in the protocol ay be m ade only. In the event of any inconsistency between this.
In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern. Changes in the protocol ay be m ade only. Web the accelerated clinical trial agreement (acta) was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting. Web sample from industry sponsor’s section [2]3 conduct of study/protocol deviations 2.1 performance of study. Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,. Site shall, and shall cause principal investigator to, diligently. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the.
Web Sample From Industry Sponsor’s Section [2]3 Conduct Of Study/Protocol Deviations 2.1 Performance Of Study.
Changes in the protocol ay be m ade only. Site shall, and shall cause principal investigator to, diligently. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. In the event of any inconsistency between this agreement and the protocol, the terms of this agreement shall govern.
Web The Accelerated Clinical Trial Agreement (Acta) Was Prepared With The Intent To Facilitate Relationships With Industry Sponsors That Are Interested In Expediting.
Web the three major components of a clinical trial agreement are (1) clinical trial details (location, date, participants, sponsor support, etc), terms and conditions (termination,.