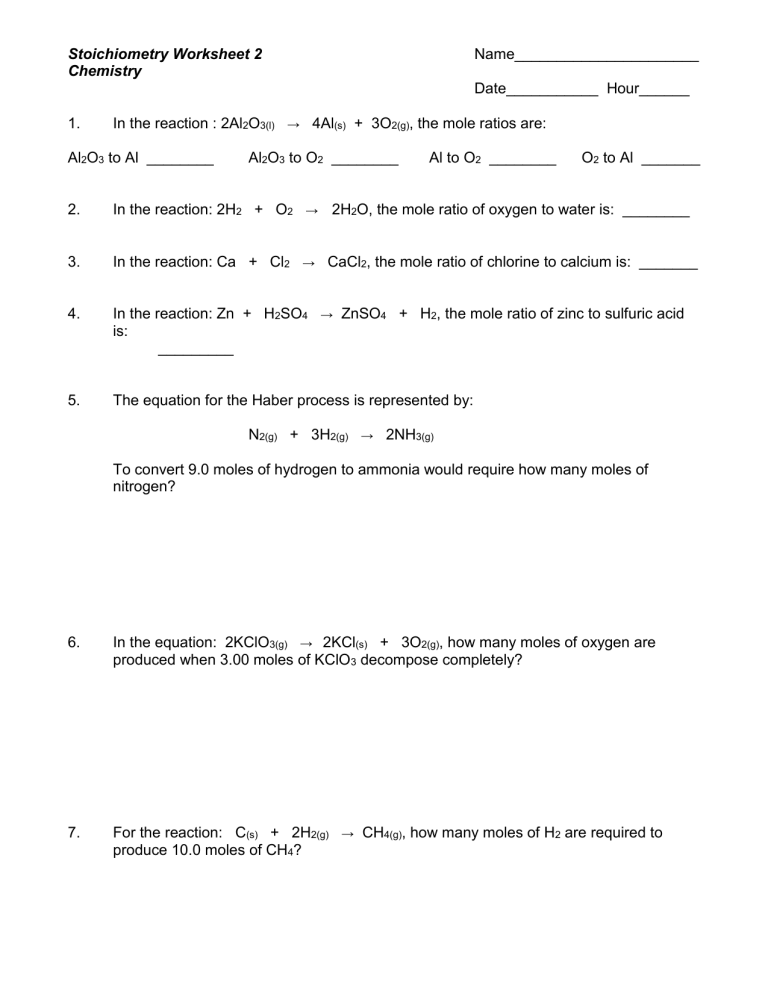
Chemistry Stoichiometry Worksheet Answers - This video is an introduction to. 154.8 g naoh b how many grams of na2o are. How many grams of naoh is produced from 1.20 x 102 grams of na2o? Web stoichiometry exercises stoichiometry exercises cristelgil288 member for 2 years 8 months age: Web stoichiometry google classroom introduction you might use stoichiometry skills to double a cookie recipe! 1 stoichiometry calculation practice worksheet. Web balancing, stoichiometry, and nomenclature grade/level: Web once we know the numbers of moles, we can use the relationships between moles and molar masses of the various. Given the equation 3a + b → c + d, you react 1 mole of a with 3 moles of b. Silver and nitric acid react according to the following balanced equation:
Printable Stoichiometry Worksheet Answers Yes Chemistry worksheets
Silver and nitric acid react according to the following balanced equation: 3 ag(s) + 4 hno 3 (aq) 3 agno 3. Web this is a series of lectures in videos covering chemistry topics taught in high schools. Web stoichiometry (worksheet) last updated. Christopherson s t o i c h i o m e t r y :
Chemical Equations And Stoichiometry Worksheet Answers Worksheet List
Calculate the number of moles of naoh that are needed to react. Remember to pay careful attention to what you are given, and what you are trying to find. Web this is a series of lectures in videos covering chemistry topics taught in high schools. Web stoichiometry google classroom introduction you might use stoichiometry skills to double a cookie recipe!.
Stoichiometry Worksheet Answer Key Promotiontablecovers
Web this is a series of lectures in videos covering chemistry topics taught in high schools. Christopherson s t o i c h i o m e t r y : Web the following flow chart may help you work stoichiometry problems. Remember to pay careful attention to what you are given, and what you are trying to find. 2.
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
Web stoichiometry practice worksheet: Worked solutions answer the following questions on your own paper. 3 ag(s) + 4 hno 3 (aq) 3 agno 3. Remember to pay careful attention to what you are given, and what you are trying to find. Web stoichiometry exercises stoichiometry exercises cristelgil288 member for 2 years 8 months age:
Gas Stoichiometry Worksheet With Solutions —
Web problem silver is often extracted from ores as k [ag (cn) 2] and then recovered by the reaction how many. Christopherson s t o i c h i o m e t r y : 154.8 g naoh b how many grams of na2o are. Calculate the number of moles of naoh that are needed to react. Given the.
13 Best Images of Chemistry Mole Worksheet Mole Avogadro Number
Web stoichiometry exercises stoichiometry exercises cristelgil288 member for 2 years 8 months age: Web this is a stoichiometry worksheet package for grade 11 chemistry. Worked solutions answer the following questions on your own paper. Christopherson s t o i c h i o m e t r y : Web stoichiometry (worksheet) last updated.
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
Web once we know the numbers of moles, we can use the relationships between moles and molar masses of the various. How many grams of naoh is produced from 1.20 x 102 grams of na2o? Calculate the number of moles of naoh that are needed to react. It includes 11 worksheets, with answers,. Web stoichiometry (worksheet) last updated.
10 Best Images of Stoichiometry Worksheet 2 Answer Key Chemistry
Web this is a stoichiometry worksheet package for grade 11 chemistry. Silver and nitric acid react according to the following balanced equation: Web problem silver is often extracted from ores as k [ag (cn) 2] and then recovered by the reaction how many. Worked solutions answer the following questions on your own paper. 2 kclo3 2 kcl + 3 02.
Gas Stoichiometry Worksheet Doc worksheet today
Web stoichiometry (worksheet) last updated. Web stoichiometry (worksheet) solutions: Calculate the number of moles of naoh that are needed to react with 500.0 g of h 2 so 4 according to the following equation: How many grams of naoh is produced from 1.20 x 102 grams of na2o? Calculate the number of moles of naoh that are needed to react.
APChemistry Stoichiometry Practice Problems with Answers. Mole
Worked solutions answer the following questions on your own paper. Web stoichiometry exercises stoichiometry exercises cristelgil288 member for 2 years 8 months age: Web the following flow chart may help you work stoichiometry problems. Web this is a series of lectures in videos covering chemistry topics taught in high schools. Christopherson s t o i c h i o m.
Web once we know the numbers of moles, we can use the relationships between moles and molar masses of the various. 3 ag(s) + 4 hno 3 (aq) 3 agno 3. Christopherson s t o i c h i o m e t r y : Web this is a stoichiometry worksheet package for grade 11 chemistry. Web the following flow chart may help you work stoichiometry problems. Web stoichiometry practice worksheet: The most fun you can have with a calculator. Calculate the number of moles of naoh that are needed to react. 154.8 g naoh b how many grams of na2o are. H 2 so 4 + 2 naoh na 2 so 4 + 2 h. How many grams of naoh is produced from 1.20 x 102 grams of na2o? Silver and nitric acid react according to the following balanced equation: Web balancing, stoichiometry, and nomenclature grade/level: Web stoichiometry (worksheet) solutions: Web this is a series of lectures in videos covering chemistry topics taught in high schools. Web stoichiometry google classroom introduction you might use stoichiometry skills to double a cookie recipe! 2 kclo3 2 kcl + 3 02 how many moles ofo 2 can be. It includes 11 worksheets, with answers,. Calculate the number of moles of naoh that are needed to react with 500.0 g of h 2 so 4 according to the following equation: Web the enclosed video worksheet (crash course chemistry video worksheet 6:
Web This Is A Series Of Lectures In Videos Covering Chemistry Topics Taught In High Schools.
Christopherson s t o i c h i o m e t r y : Web problem silver is often extracted from ores as k [ag (cn) 2] and then recovered by the reaction how many. Web balancing, stoichiometry, and nomenclature grade/level: 2 kclo3 2 kcl + 3 02 how many moles ofo 2 can be.
Remember To Pay Careful Attention To What You Are Given, And What You Are Trying To Find.
1 stoichiometry calculation practice worksheet. The most fun you can have with a calculator. Calculate the number of moles of naoh that are needed to react with 500.0 g of h 2 so 4 according to the following equation: It includes 11 worksheets, with answers,.
Calculate The Number Of Moles Of Naoh That Are Needed To React.
Silver and nitric acid react according to the following balanced equation: Web stoichiometry exercises stoichiometry exercises cristelgil288 member for 2 years 8 months age: Web the enclosed video worksheet (crash course chemistry video worksheet 6: Given the equation 3a + b → c + d, you react 1 mole of a with 3 moles of b.
Web The Following Flow Chart May Help You Work Stoichiometry Problems.
H 2 so 4 + 2 naoh na 2 so 4 + 2 h. 3 ag(s) + 4 hno 3 (aq) 3 agno 3. Web once we know the numbers of moles, we can use the relationships between moles and molar masses of the various. Worked solutions answer the following questions on your own paper.