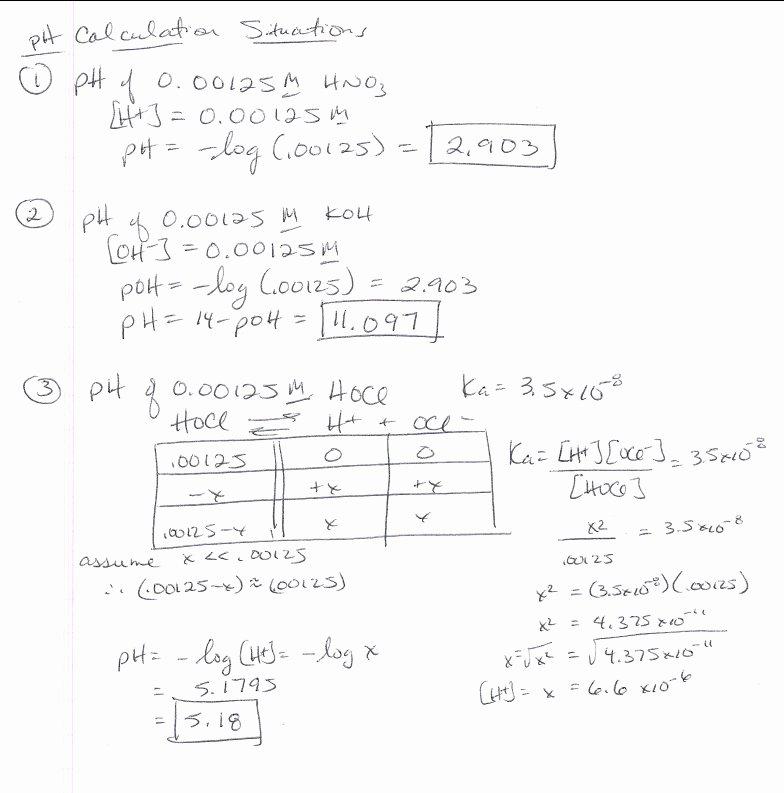
Chemistry Ph And Poh Worksheet Answer Key - Encouraged to help my personal weblog,. Fill in the missing information in the table below. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web answer the lower the ph, the higher the acidity. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Fill in the missing information in the table below. Web google classroom definitions of ph, poh, and the ph scale. At this temperature, then, neutral solutions exhibit ph = poh = 6.31,. Ph and poh calculations part 1:
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Find the [hsc ] in. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2. Web ph and poh worksheet answer key.
Print Free pH Practice Worksheets Worksheets, Chemistry and School
Includes full solutions and score reporting. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2. Web crash course chemistry #30 (ph and poh) worksheet. For each of the.
Ph And Poh Worksheet Answer Key Askworksheet
Encouraged to help my personal weblog,. Web google classroom definitions of ph, poh, and the ph scale. Fill in the missing information in the table below. For each of the problems. At this temperature, then, neutral solutions exhibit ph = poh = 6.31,.
Ph And Poh Calculations Worksheet Answer Key Thekidsworksheet
Web 14.00 = ph + poh. At this temperature, then, neutral solutions exhibit ph = poh = 6.31,. Fill in the missing information in the table below. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. [h+] = 4.29 × 10−11 m calculate [h+] in each of the.
Ph and Poh Worksheet
Encouraged to help my personal weblog,. Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2. Web answer the lower the ph, the higher the acidity. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). Web this downloadable pdf worksheet is for students to practice.
Chemistry Ph Worksheet Answer Key Auhsd + My PDF Collection 2021
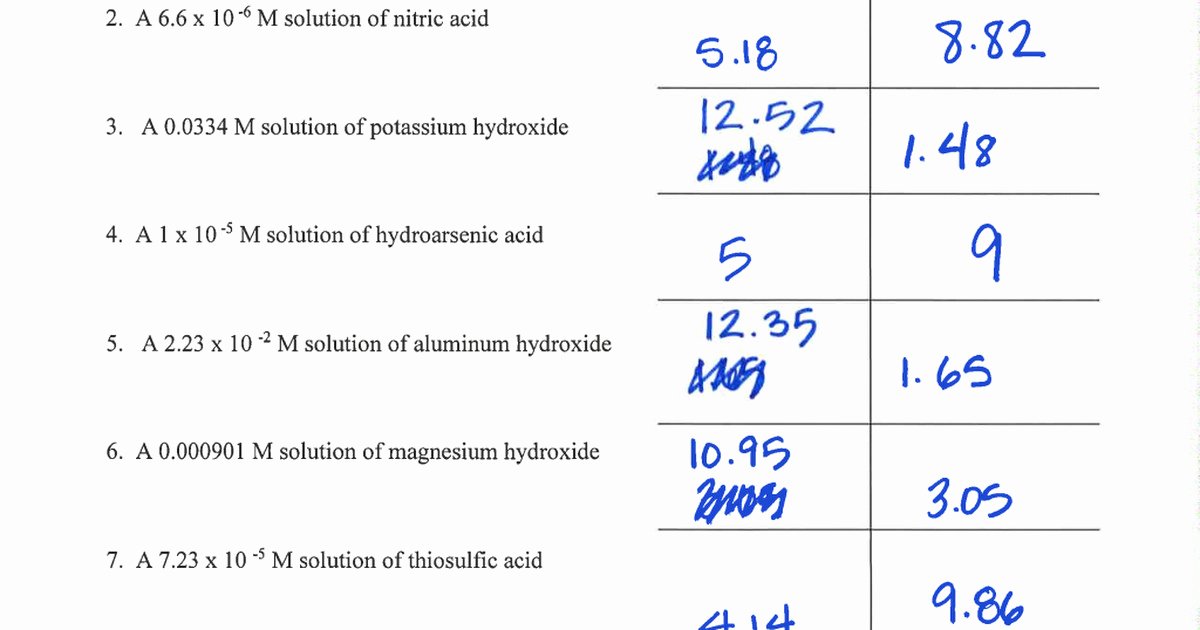
Find the [hsc ] in. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Fill in the missing information in the table below. Poh = 4.95 calculate [oh−] in. Web answer the lower the ph, the higher the acidity.
8.3 pH and pOH Chemistry for Chemical Engineers
Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! For each of the problems. Ph and poh calculations part 1: As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. At this temperature, then, neutral solutions exhibit ph = poh = 6.31,.
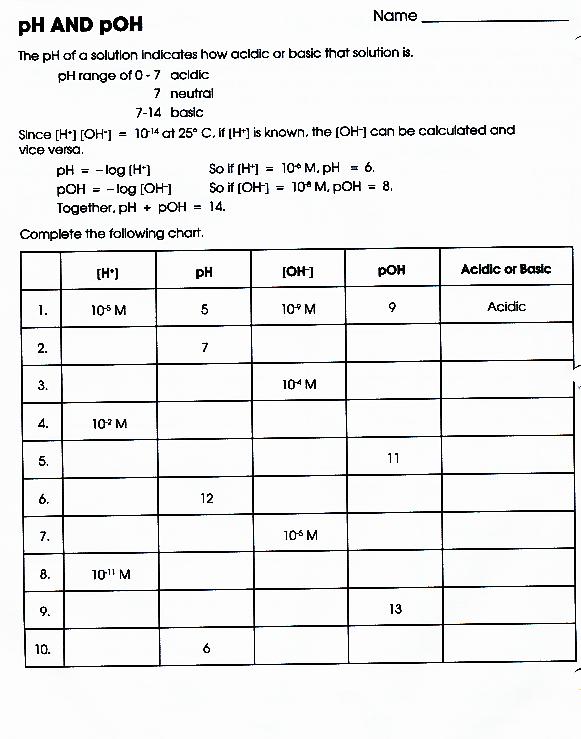
pH and pOH Practice Worksheet
Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Includes full solutions and score reporting. Web 14.00 = ph + poh. Web ph and poh worksheet answer key.
Ph and Poh Worksheet
Encouraged to help my personal weblog,. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Fill in the missing information in the table below. Web ph and poh worksheet answer key riz books from rizbooks.blogspot.com. Calculating the ph of a strong acid or base solution.
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2. Fill in the missing information in the table below. Ph and poh calculations part 1: Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Ph and poh wks answers author:
Web google classroom definitions of ph, poh, and the ph scale. Fill in the missing information in the table below. 4/7/2015 9:57:16 am keywords () Calculating the ph of a strong acid or base solution. Web 14.00 = ph + poh. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Ph and poh calculations part 1: [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web answer the lower the ph, the higher the acidity. Fill in the missing information in the table below. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). At this temperature, then, neutral solutions exhibit ph = poh = 6.31,. Fill in the missing information in the table below. For each of the problems. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Ph and poh wks answers author: Web solution from equation 15.8.3, ph + poh = 14.00. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Find the [hsc ] in. Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2.
Poh = 4.95 Calculate [Oh−] In.
Ph and poh calculations part 1: From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Fill in the missing information in the table below.
Ph And Poh Wks Answers Author:
Encouraged to help my personal weblog,. Fill in the missing information in the table below. 4/7/2015 9:57:16 am keywords () Web ph and poh worksheet answer key riz books from rizbooks.blogspot.com.
Web Answer The Lower The Ph, The Higher The Acidity.
Web solution from equation 15.8.3, ph + poh = 14.00. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Therefore, (a) ph = 9.8 < (d) ph = 6.4 < (c) ph = 4.7 < (b) ph = 1.2 2. For each of the problems.
Find The [Hsc ] In.
Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web google classroom definitions of ph, poh, and the ph scale. Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Includes full solutions and score reporting.






/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)