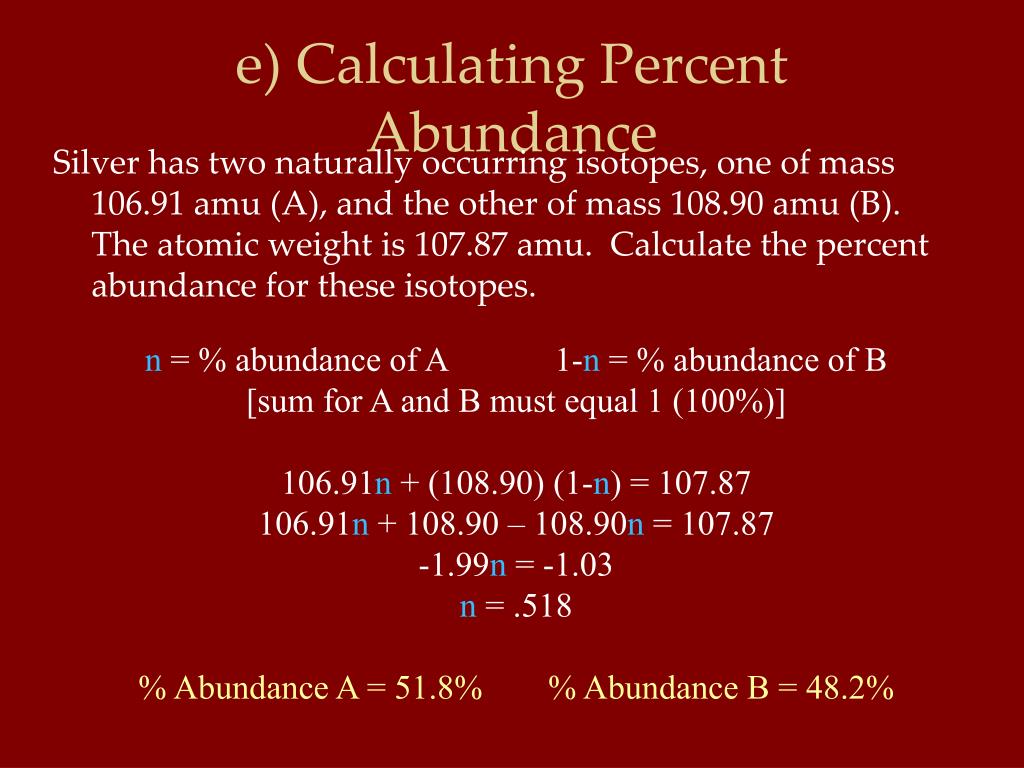
Calculating Percent Abundance Of Isotopes Worksheet - Set up the relative abundance. Structured to start by thinking of. Web how to calculate the percent abundance of an isotope step 1: Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: A scaffolded worksheet giving students practise in calculating relative. Web worksheet to help students calculate ram from relative abundances of isotopes. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web chrome_reader_mode enter reader output. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Find the average atomic mass step 2:
How to Solve for Percent Abundance of Isotopes Examples, Practice
Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Web chrome_reader_mode enter reader output. Mass of isotope % abundance 36.96590. Web this relative isotopies abundance in.
How To Calculate Percent Abundance Of 2 Isotopes
Web to calculate a weighted average of its isotope masses. Web how to calculate the percent abundance of an isotope step 1: Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. Find the average atomic mass step 2: Web atomic mass = ( 11.934 ) + (.
Subatomic Particles And Isotopes Worksheets Answers Word problem
Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Use the equation for atomic mass to complete the following problems. Web the relativist isotope abundance in chemistry can the.
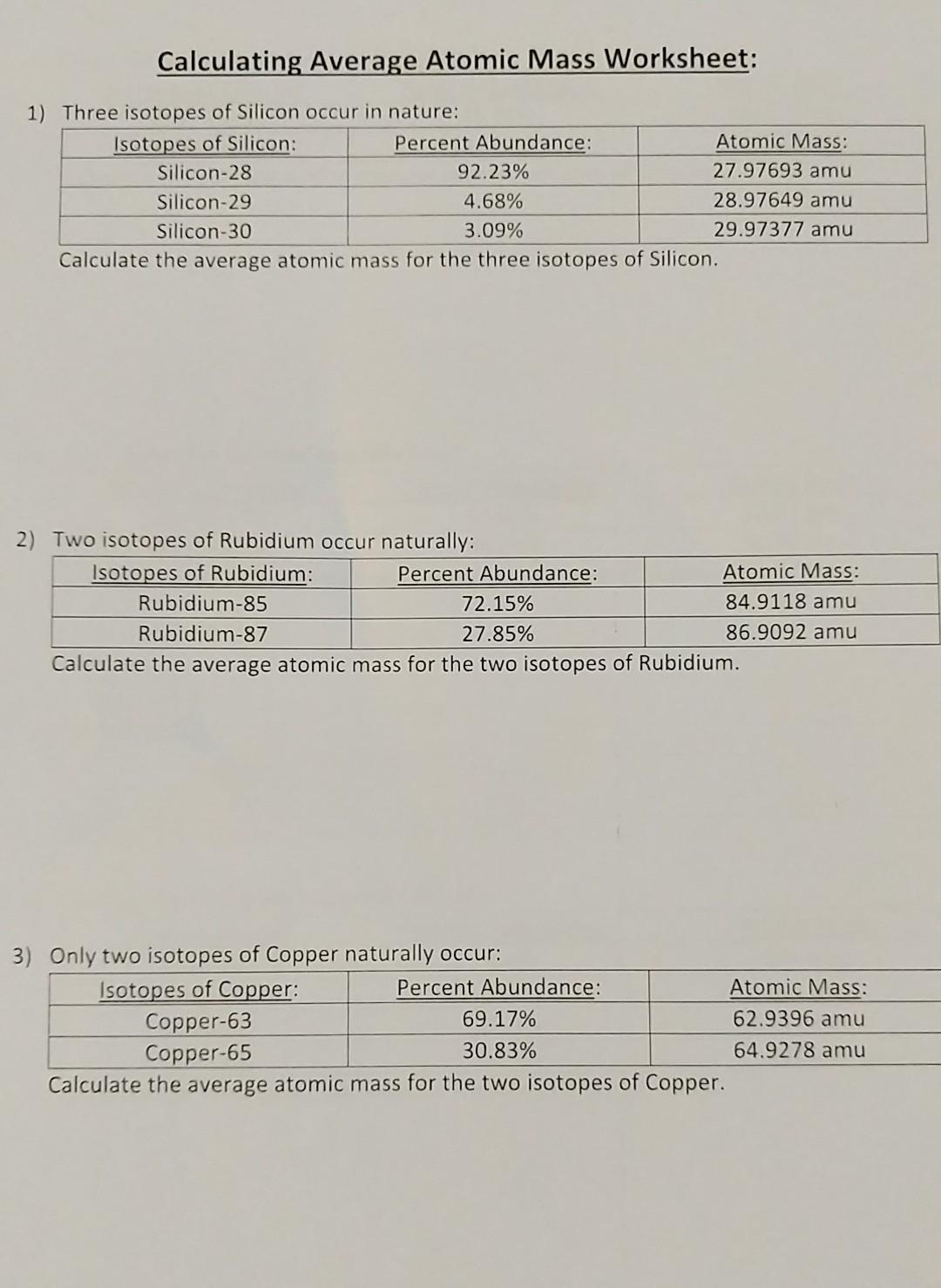
Solved Calculating Average Atomic Mass Worksheet 1) Three
Web abundance and the masses for these two isotopes. Web chrome_reader_mode enter reader output. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Web in a naturally occurring element, the fractional abundance is the percentage of.
Calculating Average Atomic Mass Worksheet
Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Mass of isotope % abundance 36.96590. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Find the average atomic mass.
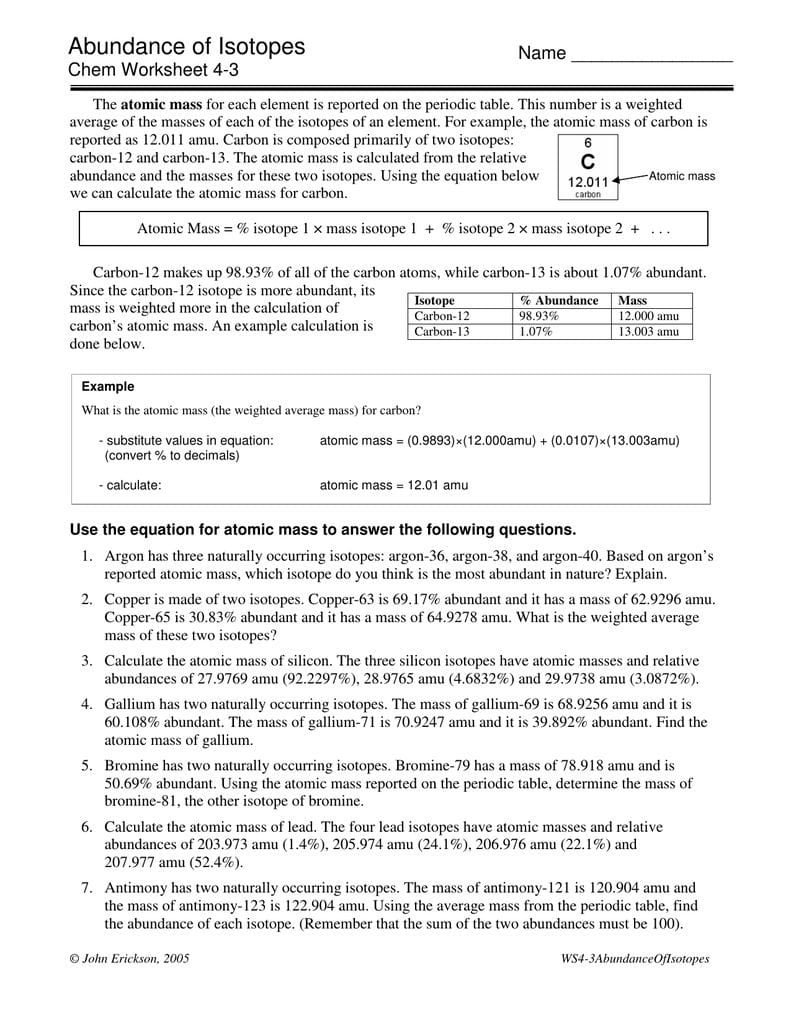
Abundance Of Isotopes Chem Worksheet 4 3 Answers —
Web i'll show you how to use the above example of nitrogen. Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Web chrome_reader_mode enter reader output. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. Web to calculate a weighted.
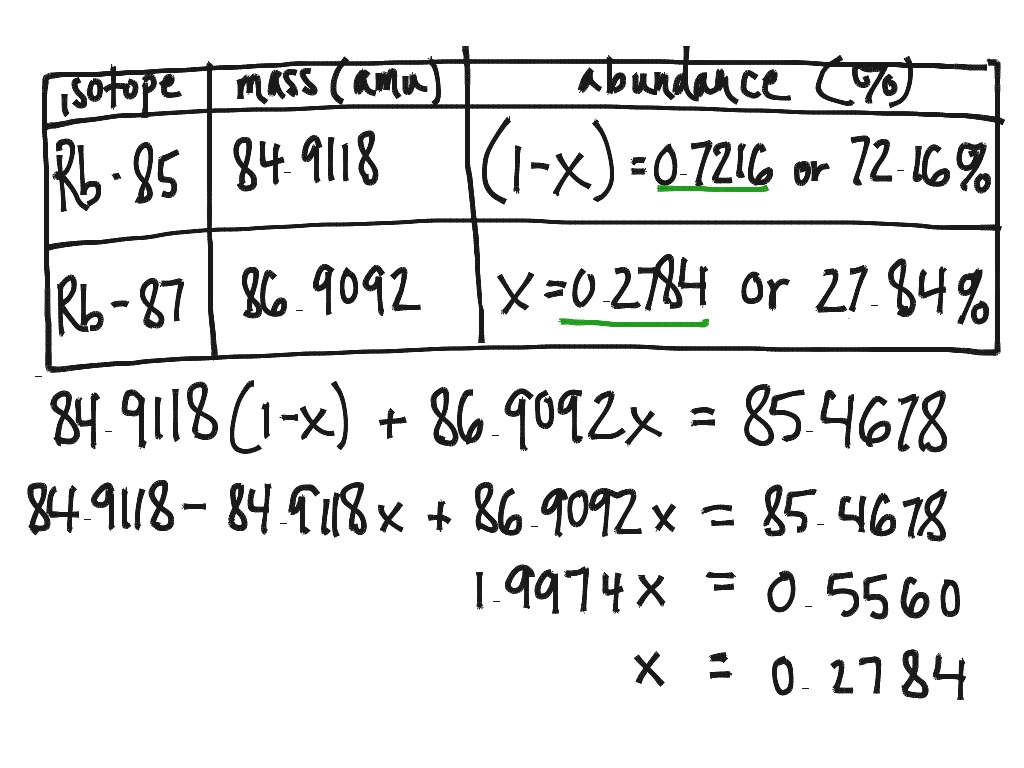
Average Atomic Mass and Percent Abundance Worksheet 2 and KEY Isotope
Mass of isotope % abundance 36.96590. Using the equation below we can calculate the atomic mass for carbon. Using the atomic mass on the. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element.
Calculating Percentage abundance of each isotope YouTube
Web to calculate a weighted average of its isotope masses. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Set up the relative abundance. Web chrome_reader_mode enter reader output.
Calculating natural abundance Colororient
Using the atomic mass on the. Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Set up the relative abundance. Mass of isotope % abundance 36.96590.
ShowMe percent abundance
Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Structured to start by thinking of..
Web abundance and the masses for these two isotopes. Web worksheet to help students calculate ram from relative abundances of isotopes. Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written. Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Use the equation in question 1 to calculate the atomic mass of an element. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Set up the relative abundance. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. A scaffolded worksheet giving students practise in calculating relative. Structured to start by thinking of. Web i'll show you how to use the above example of nitrogen. Find the average atomic mass step 2: Using the atomic mass on the. Web chrome_reader_mode enter reader output. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in. Using the equation below we can calculate the atomic mass for carbon. Use the equation for atomic mass to complete the following problems.
Web The Absolute Isotope Abundance In Chemistry Is Aforementioned Percentage Of One Particular Isotope That Occurring In Nature.
Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring. Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows.
Use The Equation For Atomic Mass To Complete The Following Problems.
Structured to start by thinking of. Using the equation below we can calculate the atomic mass for carbon. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Use the equation in question 1 to calculate the atomic mass of an element.
Web I'll Show You How To Use The Above Example Of Nitrogen.
Mass of isotope % abundance 36.96590. Web in a naturally occurring element, the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms, written. Find the average atomic mass step 2: Using the atomic mass on the.
Once We Collect The Relative Masses Of Each Isotope From Mass Spectrometry Data, We Can Use This.
Set up the relative abundance. A scaffolded worksheet giving students practise in calculating relative. Web atomic mass = ( 11.934 ) + ( 0.077 ) = 12.011 amu directions: Web abundance and the masses for these two isotopes.