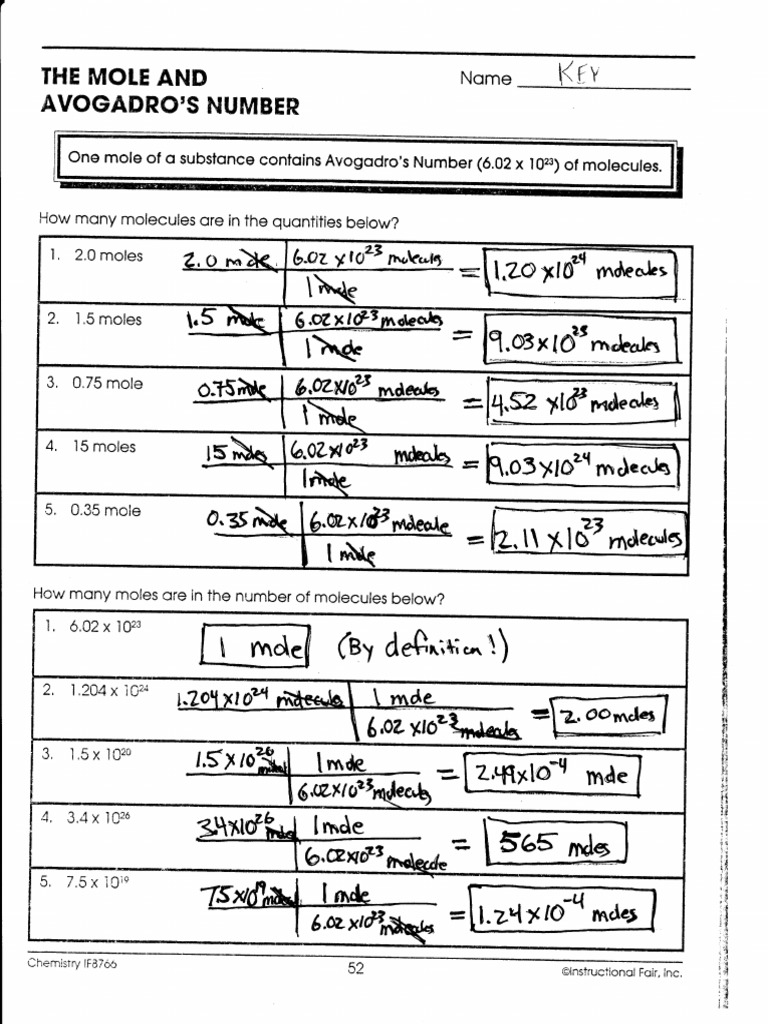
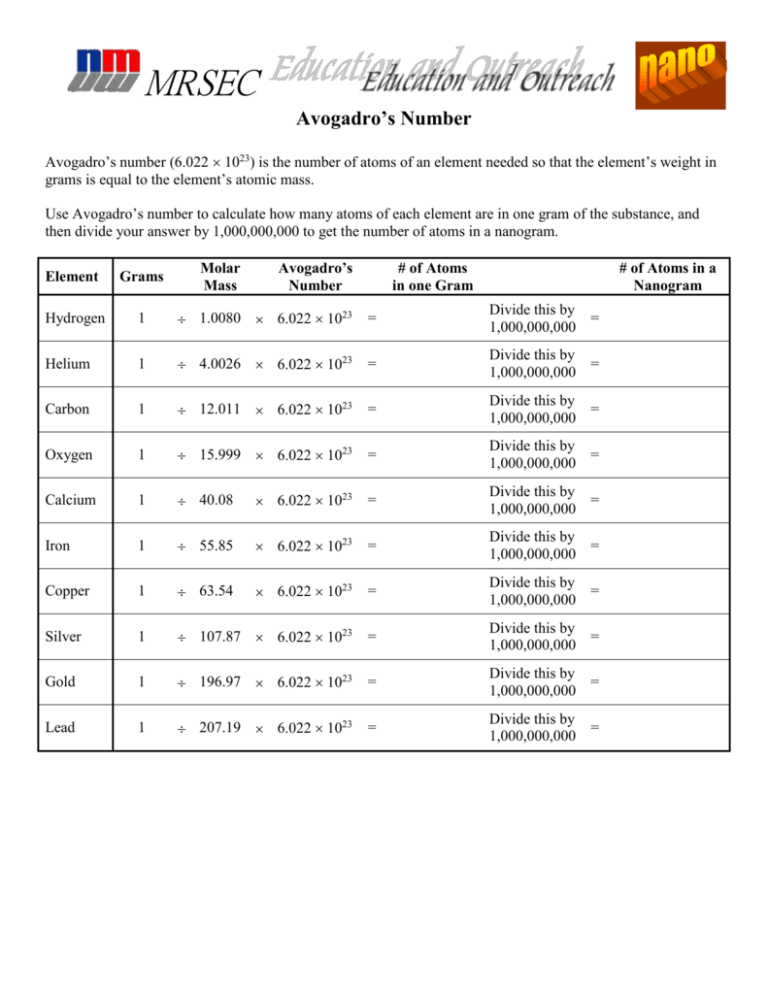
Avogadro's Number Worksheet - Questions from the powerpoint lecture: Using the information provided on slide #3, a) 1 mole of any. Determine the number of representative particles in each of the following. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Number of lithium ions, sulfate, ions, s atoms, and o. For most purposes it is rounded off to 6.022 1023. Web the number 6.022 137 1023 is called avogadro’s number. Because this is an awkward number to write over and over. Calculate each of the following quantities. Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he =.
avogadro's number worksheet
For most purposes it is rounded off to 6.022 1023. Because this is an awkward number to write over and over. Using the information provided on slide #3, a) 1 mole of any. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Determine the number of representative particles in each of the following.
The Mole And Avogadro's Number Worksheet pivotinspire
Questions from the powerpoint lecture: Percent mass, moles, and avogadros number. Web the number 6.022 137 1023 is called avogadro’s number. Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he =. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams.
The Mole And Avogadro's Number Worksheet Answers richinspire
Using the information provided on slide #3, a) 1 mole of any. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Determine the number of representative particles in each of the following. Web regents chemistry chapter 7.1 avogadro’s number lecture worksheet. Percent mass, moles, and avogadros number.
[Solved] Estimating Avogadro's Number Activity 1 Data Table 1 Questions
Determine the number of representative particles in each of the following. Percent mass, moles, and avogadros number. Using the information provided on slide #3, a) 1 mole of any. Because this is an awkward number to write over and over. Calculate each of the following quantities.
Avogadro's Number And The Mole Worksheets
Number of lithium ions, sulfate, ions, s atoms, and o. Determine the number of representative particles in each of the following. Percent mass, moles, and avogadros number. For most purposes it is rounded off to 6.022 1023. Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he.
The Mole And Avogadro's Number Worksheet
Percent mass, moles, and avogadros number. Questions from the powerpoint lecture: Calculate each of the following quantities. Web the number 6.022 137 1023 is called avogadro’s number. Because this is an awkward number to write over and over.
AVOGADRO'S NUMBER WORKSHEET
Questions from the powerpoint lecture: Number of lithium ions, sulfate, ions, s atoms, and o. Web regents chemistry chapter 7.1 avogadro’s number lecture worksheet. Using the information provided on slide #3, a) 1 mole of any. Calculate each of the following quantities.
The Mole And Avogadro's Number Worksheet
Web the number 6.022 137 1023 is called avogadro’s number. Calculate each of the following quantities. For most purposes it is rounded off to 6.022 1023. Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he =. Percent mass, moles, and avogadros number.
Avogadro's Number Worksheet
Questions from the powerpoint lecture: Because this is an awkward number to write over and over. Calculate each of the following quantities. Web regents chemistry chapter 7.1 avogadro’s number lecture worksheet. Percent mass, moles, and avogadros number.
Worksheet Moles, Molecules & STP KEY
Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he =. Web the number 6.022 137 1023 is called avogadro’s number. Determine the number of representative particles in each of the following. Because this is an awkward number to write over and over. Number of lithium ions,.
Web the number 6.022 137 1023 is called avogadro’s number. Because this is an awkward number to write over and over. Number of lithium ions, sulfate, ions, s atoms, and o. For most purposes it is rounded off to 6.022 1023. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Therefore, 6.02 10 23 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d) he =. Using the information provided on slide #3, a) 1 mole of any. Determine the number of representative particles in each of the following. Web regents chemistry chapter 7.1 avogadro’s number lecture worksheet. Percent mass, moles, and avogadros number. Calculate each of the following quantities. Questions from the powerpoint lecture:
Therefore, 6.02 10 23 Atoms Of (A) Cu = 63.55 G (C) S = 32.07 G (B) Hg = 200.59 G (D) He =.
Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Percent mass, moles, and avogadros number. Because this is an awkward number to write over and over. Number of lithium ions, sulfate, ions, s atoms, and o.
Web Regents Chemistry Chapter 7.1 Avogadro’s Number Lecture Worksheet.
Web the number 6.022 137 1023 is called avogadro’s number. Using the information provided on slide #3, a) 1 mole of any. Questions from the powerpoint lecture: Determine the number of representative particles in each of the following.
Calculate Each Of The Following Quantities.
For most purposes it is rounded off to 6.022 1023.