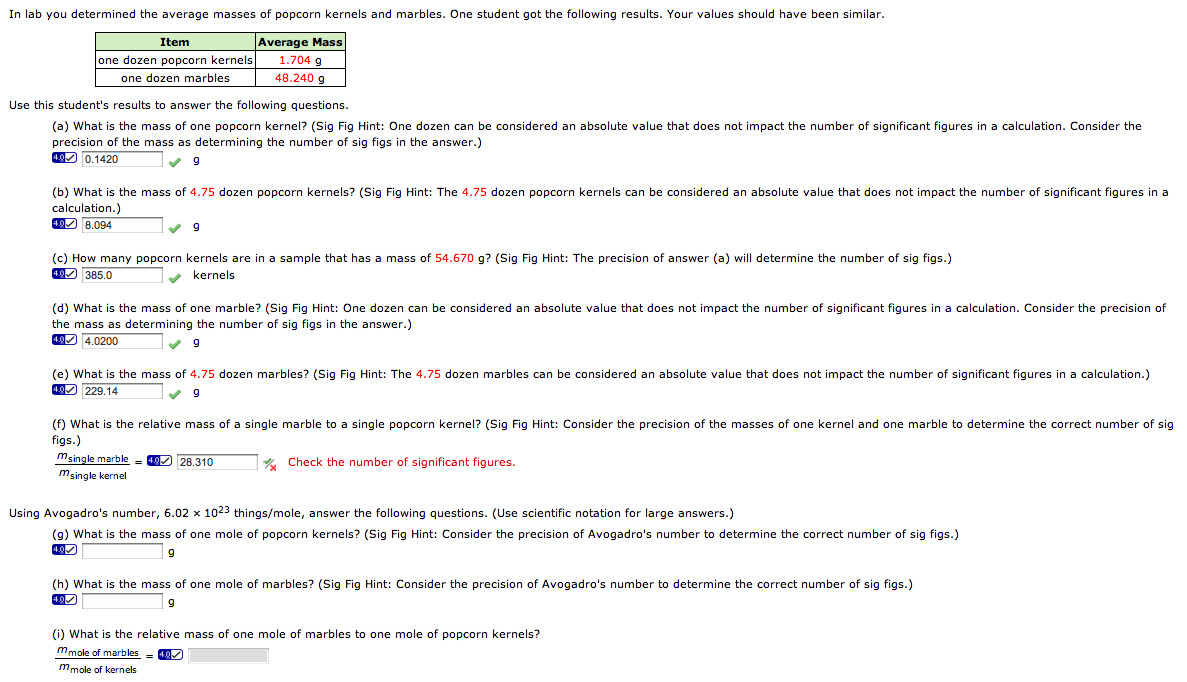
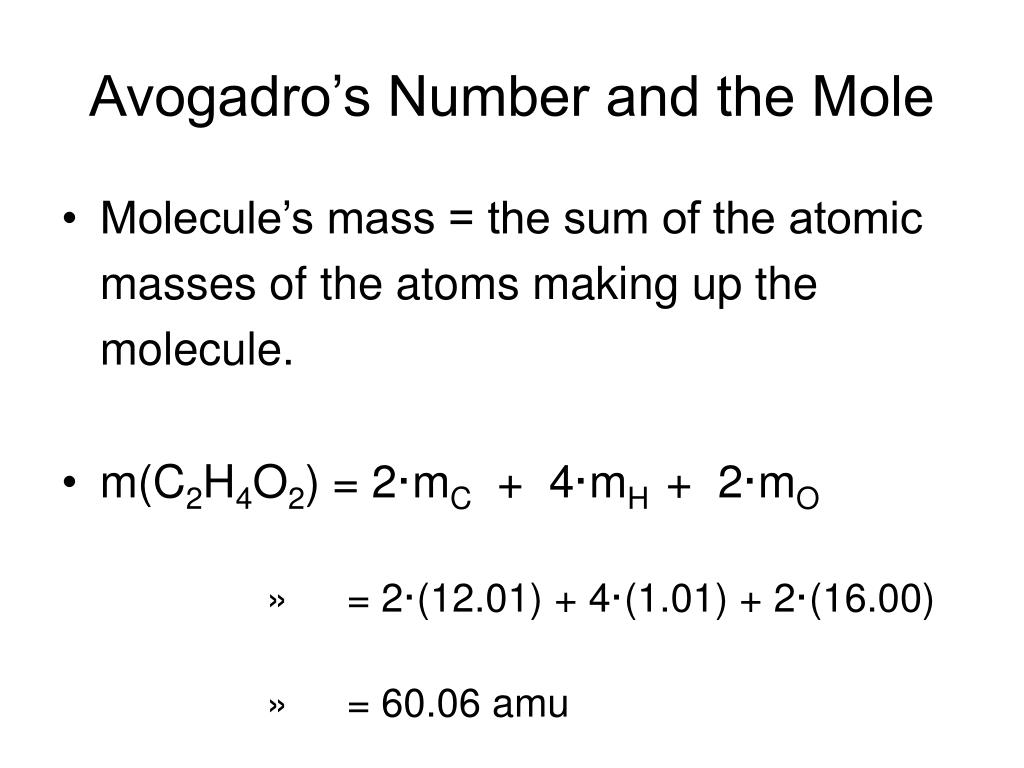Avogadro's Number And The Mole Worksheet - Web systems of objects with friction 6m. Web the mole and avogadro's number. Web moles and molar mass. A chemical species is either an atom (e.g. Relative atomic mass, as john dalton understood. Web the number 6.022 137 1023 is called avogadro’s number. Web use avogadro's number to convert to moles and vice versa given the number of particles of an element. A mole of substance is that. In chemistry, a mole is a really big number. For most calculations, a rounded value of 6.022 x 1023 (four.
Mole Problem? Chemistry Avogadro's Number Mole Day Mole Day Pin
Be able to calculate the number of moles in a. A mole of substance is that. Relative atomic mass, as john dalton understood. This number ( 6.02 x. And i'm also gonna show you a systematic.
PPT Chapter 3 PowerPoint Presentation ID258640
Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample. For most purposes it is rounded off to 6.022 1023. Web mole & avogadro’s number example question. Web use avogadro's number to convert to moles and vice versa given the number of particles of an element. Web this senior chemistry lesson.
13 Best Images of Chemistry Mole Worksheet Mole Avogadro Number
Web define avogadro's number and explain why it is important to know. Percent mass, moles, and avogadros number. Web nine problems with key on calculating number of particles in a mole or number of moles in a given number of particles using. B) the number of oxygen atoms in. Using the information in the table, calculate the number of moles.
The Mole And Avogadro's Number Worksheet
Web avogadro's number one of the most important ideas in chemistry is the mole concept. Web mole & avogadro’s number example question. Web nine problems with key on calculating number of particles in a mole or number of moles in a given number of particles using. Web so i'm going to introduce to you the moles and also avocados number.
The Mole And Avogadros Number Worksheet Answers Escolagersonalvesgui
Calculate each of the following quantities. This number ( 6.02 x. Systems of objects on inclined planes with friction 13m. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample. Web nine problems with key on calculating number of particles in a mole or number of moles in a given number.
The Mole And Avogadro's Number Worksheet
For most purposes it is rounded off to 6.022 1023. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). Relative atomic mass, as john dalton understood. And i'm also gonna show you a systematic. For most calculations, a rounded value of 6.022 x 1023 (four.
The Mole And Avogadro S Number Worksheet Answers worksheet
Web moles and molar mass. Calculate each of the following quantities. A chemical species is either an atom (e.g. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following. Web avogadro's number one of the most important ideas in chemistry is the mole concept.
20 Avogadro Number Worksheet Answers Worksheet From Home
Web moles and molar mass. For most purposes it is rounded off to 6.022 1023. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample. Calculate each of the following quantities. A) the mass in mg of mol ag and.
20 Avogadro Number Worksheet Answers Worksheet From Home
Percent mass, moles, and avogadros number. For most purposes it is rounded off to 6.022 1023. Web the number 6.022 137 1023 is called avogadro’s number. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following. And i'm also gonna show you a systematic.
AVOGADRO'S NUMBER WORKSHEET
A mole of substance is that. In chemistry, a mole is a really big number. Web using avogadro’s number to convert from molecules or atoms to moles. Web systems of objects with friction 6m. Web the number 6.022 137 1023 is called avogadro’s number.
For most purposes it is rounded off to 6.022 1023. Web the mole and avogadro's number. Calculate each of the following quantities. A chemical species is either an atom (e.g. Web this compact collection of slides explains the history and use of the mole unit. Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). Web moles and molar mass. Web introducing moles and avogadro’s number. And i'm also gonna show you a systematic. Web state the mass of avogadro’s number of atoms for each of the following elements: In chemistry, a mole is a really big number. Web avogadro's number one of the most important ideas in chemistry is the mole concept. Be able to calculate the number of moles in a. A) the mass in mg of mol ag and. Web so i'm going to introduce to you the moles and also avocados number in this video. A mole of substance is that. Web mole & avogadro’s number example question. Web use avogadro's number to convert to moles and vice versa given the number of particles of an element. Systems of objects on inclined planes with friction 13m.
Web Use Avogadro's Number To Convert To Moles And Vice Versa Given The Number Of Particles Of An Element.
Web moles and molar mass. A chemical species is either an atom (e.g. B) the number of oxygen atoms in. Web using avogadro’s number to convert from molecules or atoms to moles.
For Most Purposes It Is Rounded Off To 6.022 1023.
Be able to calculate the number of moles in a. Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice. Web systems of objects with friction 6m. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions).
Systems Of Objects On Inclined Planes With Friction 13M.
Web the mole and avogadro's number. A mole of substance is that. Web introducing moles and avogadro’s number. Web state the mass of avogadro’s number of atoms for each of the following elements:
Web Avogadro's Number One Of The Most Important Ideas In Chemistry Is The Mole Concept.
Web so i'm going to introduce to you the moles and also avocados number in this video. Web mole & avogadro’s number example question. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample. Web define avogadro's number and explain why it is important to know.